In Part 3, the discussion focuses on fitting techniques to use on cases which are out of the norm. Those difficult cases that occur when there is an increased level of distortion in the auditory system which are not typically covered in research or clinical literature. 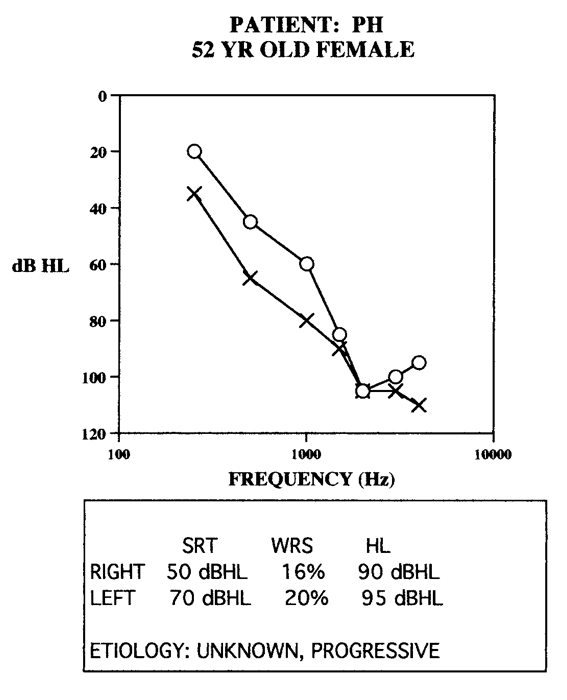
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pYnxEs
Αρχειοθήκη ιστολογίου
-
►
2023
(269)
- ► Φεβρουαρίου (133)
- ► Ιανουαρίου (136)
-
►
2022
(2046)
- ► Δεκεμβρίου (165)
- ► Σεπτεμβρίου (161)
- ► Φεβρουαρίου (165)
-
►
2021
(3028)
- ► Δεκεμβρίου (135)
- ► Σεπτεμβρίου (182)
- ► Φεβρουαρίου (324)
-
►
2020
(1051)
- ► Δεκεμβρίου (292)
- ► Σεπτεμβρίου (60)
- ► Φεβρουαρίου (28)
-
►
2019
(2277)
- ► Δεκεμβρίου (18)
- ► Σεπτεμβρίου (54)
- ► Φεβρουαρίου (89)
-
▼
2018
(26280)
- ► Δεκεμβρίου (189)
-
▼
Απριλίου
(5246)
-
▼
Απρ 01
(144)
- Complex versus Standard Fittings: Part 3
- No association between circulating concentrations ...
- Pembrolizumab for primary malignant melanoma of th...
- Myeloma Cells Are Activated in Bone Marrow Microen...
- Integrative Genomic Analysis Predicts Causative Ci...
- In Silico Evaluation of Pharmacokinetic Optimizati...
- Pyruvate Dehydrogenase PDH-E1{beta} Controls Tumor...
- Fc-Mediated Anomalous Biodistribution of Therapeut...
- Polyol Pathway Links Glucose Metabolism to the Agg...
- STAT3/PIAS3 Levels Serve as “Early Signature” Gene...
- Keratin 19 Expression in Hepatocellular Carcinoma ...
- TRIM59 Promotes Gliomagenesis by Inhibiting TC45 D...
- MBD2 Ablation Impairs Lymphopoiesis and Impedes Pr...
- Selective mTORC2 Inhibitor Therapeutically Blocks ...
- Forkhead Box F2 Suppresses Gastric Cancer through ...
- Highlights from Recent Cancer Literature
- CCR5 Governs DNA Damage Repair and Breast Cancer S...
- miR-508 Defines the Stem-like/Mesenchymal Subtype ...
- Antiestrogen Therapy Increases Plasticity and Canc...
- Metformin-Induced Reduction of CD39 and CD73 Block...
- Downregulation of Membrane Trafficking Proteins an...
- PHD3 Controls Lung Cancer Metastasis and Resistanc...
- Tumor-Stroma IL1{beta}-IRAK4 Feedforward Circuitry...
- A Next-Generation Chimeric Antigen Receptor Induce...
- Lutetium Lu 177 Dotatate Approved by FDA [News in ...
- Noted [News in Brief]
- NIH Offers Funding for Genome-Editing Projects [Ne...
- Convergent Therapeutic Strategies to Overcome the ...
- Drug Combo Bests Sunitinib in RCC [News in Brief]
- The Oncolytic Adenovirus DNX-2401 Has Antitumor Ac...
- BMI Can Underestimate Breast Cancer Risk [News in ...
- Advances on the BRAF Front in Colorectal Cancer [I...
- Axitinib plus Pembrolizumab Is Effective in Renal ...
- MEF2C Phosphorylation Is Required for Chemotherapy...
- The PI3K{alpha} Inhibitor Alpelisib Has Activity i...
- Asparagine Bioavailability Drives Breast Cancer Me...
- AR Inhibition Achieves Responses in AR+ Triple-Neg...
- SETD1A Interacts with Cyclin K to Promote Leukemia...
- SLFN11 Blocks DNA Replication Independently of ATR...
- MEK Binding to KSR Promotes Allosteric Activation ...
- TGF{beta} Promotes Immune Evasion to Limit the Eff...
- Tissue-Specific Immunoregulation: A Call for Bette...
- MYCN Amplification Promotes Enhancer Invasion in N...
- Characterizing the Potency and Impact of Carbon Io...
- Adaptive Resistance to Chemotherapy, A Multi-FAK-t...
- A Spatio-Temporal Model of Macrophage-Mediated Dru...
- Engaging Anaphase Catastrophe Mechanisms to Eradic...
- Characterization of ABBV-221, a Tumor-Selective EG...
- JAK2 Inhibitor SAR302503 Abrogates PD-L1 Expressio...
- MCT4 Expression Is a Potential Therapeutic Target ...
- Pharmacological and Structural Characterizations o...
- Highlights of This Issue
- Preclinical Evaluation of SCC244 (Glumetinib), a N...
- Wnt/{beta}-Catenin Pathway Activation Mediates Ada...
- SKLB-23bb, A HDAC6-Selective Inhibitor, Exhibits S...
- Essential Role of Polo-like Kinase 1 (Plk1) Oncoge...
- Relative Target Affinities of T-Cell-Dependent Bis...
- PIM Kinases Are a Potential Prognostic Biomarker a...
- MI130004, a Novel Antibody-Drug Conjugate Combinin...
- Impact of Chemical-Induced Mutational Load Increas...
- Genome-Wide Gene Expression Changes in the Normal-...
- Immunomodulatory Effects of Momordica charantia Ex...
- Pioglitazone Inhibits Periprostatic White Adipose ...
- The Conundrum of Omega-3 Fatty Acids in Cancer Pre...
- Accelerating the Pace of Cancer Prevention- Right Now
- Bitter Melon Prevents the Development of 4-NQO-Ind...
- Adiposity, Inflammation, and Breast Cancer Pathoge...
- A Randomized Multicenter Phase II Study of Docosah...
- Correction: Whole-Genome Sequencing of Salivary Gl...
- Oral health and chemotherapy act as cofactors in m...
- Limited incision harvest of the rectus abdominis m...
- Oropharyngeal Dysphagia Evaluation Tools in Adults...
- Lymph node cancer of the mediastinum with a putati...
- Guest Post – Down with STEMI – The OMI Manifesto b...
- RE: “Deployment And Preterm Birth Among US Army So...
- 2017 Articles of the Year, Reviewers of the Year, ...
- How to cope with food allergy symptoms?
- Oral food challenge using different target doses a...
- Alternative Choices for Anterolateral Thigh Flaps ...
- Development of Targeted Muscle Reinnervation Model...
- Distal Nerve Transfer: Perspective of Reconstructi...
- Future Perspectives in the Management of Nerve Inj...
- Is the Oblique Branch a Preferable Vascular Pedicl...
- Dynamic Quantitative Assessment of Motor Axon Spro...
- Does Cigarette Smoking Harm Microsurgical Free Fla...
- Check the Record: Remote CT Scans for Breast Flap ...
- Clinical Study of Second Branchial Cleft Anomalies
- Cavernous Sinus Aneurysm Associated With Cerebella...
- Cleft Palate Repair Using Single Flap Palatoplasty...
- Percutaneous Autologous Fat Injection Following 2-...
- Outcomes After Open Reduction With Internal Fixati...
- Cardiac Arrest and Death Attributable to the “Divi...
- Sex Difference in the Morphology of Pineal Gland i...
- Comparison of Outpatient and Inpatient Pediatric R...
- Orbital Fracture Reconstruction Using Prebent, Ana...
- Bilateral Post-Traumatic Facial Paralysis That Con...
- Posterior Fossa Re-Exploration for Recurrent Trige...
- Rare Cause of Tinnitus: Spontaneous Temporomandibu...
- Evaluation of Facial Anthropometry Using Three-Dim...
- New Rhizotomy Procedure for Primary Spasmodic Tort...
-
▼
Απρ 01
(144)
- ► Φεβρουαρίου (6130)
- ► Ιανουαρίου (7050)
-
►
2017
(33948)
- ► Δεκεμβρίου (6715)
- ► Σεπτεμβρίου (6470)
-
►
2016
(4179)
- ► Σεπτεμβρίου (638)
- ► Φεβρουαρίου (526)
- ► Ιανουαρίου (517)
Κυριακή 1 Απριλίου 2018
Complex versus Standard Fittings: Part 3
No association between circulating concentrations of vitamin D and risk of lung cancer: An analysis in 20 prospective studies in the Lung Cancer Cohort Consortium (LC3)
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2EctsuP
Pembrolizumab for primary malignant melanoma of the central nervous system
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GqoxII
Myeloma Cells Are Activated in Bone Marrow Microenvironment by the CD180/MD-1 Complex, Which Senses Lipopolysaccharide
Multiple myeloma (MM) cells acquire dormancy and drug resistance via interaction with bone marrow stroma cells (BMSC) in a hypoxic microenvironment. Elucidating the mechanisms underlying the regrowth of dormant clones may contribute to further improvement of the prognosis of MM patients. In this study, we find that the CD180/MD-1 complex, a noncanonical lipopolysaccharide (LPS) receptor, is expressed on MM cells but not on normal counterparts, and its abundance is markedly upregulated under adherent and hypoxic conditions. Bacterial LPS and anti-CD180 antibody, but not other Toll-like receptor ligands, enhanced the growth of MM cells via activation of MAP kinases ERK and JNK in positive correlation with expression levels of CD180. Administration of LPS significantly increased the number of CD180/CD138 double-positive cells in a murine xenograft model when MM cells were inoculated with direct attachment to BMSC. Knockdown of CD180 canceled the LPS response in vitro and in vivo. Promoter analyses identified IKZF1 (Ikaros) as a pivotal transcriptional activator of the CD180 gene. Both cell adhesion and hypoxia activated transcription of the CD180 gene by increasing Ikaros expression and its binding to the promoter region. Pharmacological targeting of Ikaros by the immunomodulatory drug lenalidomide ameliorated the response of MM cells to LPS in a CD180-dependent manner in vitro and in vivo. Thus, the CD180/MD-1 pathway may represent a novel mechanism of growth regulation of MM cells in a BM milieu and may be a therapeutic target of preventing the regrowth of dormant MM cells.Significance: This study describes a novel mechanism by which myeloma cells are regulated in the bone marrow, where drug resistance and dormancy can evolve after treatment, with potential therapeutic implications for treating this often untreatable blood cancer. Cancer Res; 78(7); 1766–78. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2IitnrZ
Integrative Genomic Analysis Predicts Causative Cis-Regulatory Mechanisms of the Breast Cancer-Associated Genetic Variant rs4415084
Previous genome-wide association studies (GWAS) have identified several common genetic variants that may significantly modulate cancer susceptibility. However, the precise molecular mechanisms behind these associations remain largely unknown; it is often not clear whether discovered variants are themselves functional or merely genetically linked to other functional variants. Here, we provide an integrated method for identifying functional regulatory variants associated with cancer and their target genes by combining analyses of expression quantitative trait loci, a modified version of allele-specific expression that systematically utilizes haplotype information, transcription factor (TF)–binding preference, and epigenetic information. Application of our method to a breast cancer susceptibility region in 5p12 demonstrates that the risk allele rs4415084-T correlates with higher expression levels of the protein-coding gene mitochondrial ribosomal protein S30 (MRPS30) and lncRNA RP11-53O19.1. We propose an intergenic SNP rs4321755, in linkage disequilibrium (LD) with the GWAS SNP rs4415084 (r2 = 0.988), to be the predicted functional SNP. The risk allele rs4321755-T, in phase with the GWAS rs4415084-T, created a GATA3-binding motif within an enhancer, resulting in differential GATA3 binding and chromatin accessibility, thereby promoting transcription of MRPS30 and RP11-53O19.1. MRPS30 encodes a member of the mitochondrial ribosomal proteins, implicating the role of risk SNP in modulating mitochondrial activities in breast cancer. Our computational framework provides an effective means to integrate GWAS results with high-throughput genomic and epigenomic data and can be extended to facilitate rapid functional characterization of other genetic variants modulating cancer susceptibility.Significance: Unification of GWAS results with information from high-throughput genomic and epigenomic profiles provides a direct link between common genetic variants and measurable molecular perturbations. Cancer Res; 78(7); 1579–91. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2EdllhA
In Silico Evaluation of Pharmacokinetic Optimization for Antimitogram-Based Clinical Trials
Antimitograms are prototype in vitro tests for evaluating chemotherapeutic efficacy using patient-derived primary cancer cells. These tests might help optimize treatment from a pharmacodynamic standpoint by guiding treatment selection. However, they are technically challenging and require refinements and trials to demonstrate benefit to be widely used. In this study, we performed simulations aimed at exploring how to validate antimitograms and how to complement them by pharmacokinetic optimization. A generic model of advanced cancer, including pharmacokinetic–pharmacodynamic monitoring, was used to link dosing schedules with progression-free survival (PFS), as built from previously validated modules. This model was used to explore different possible situations in terms of pharmacokinetic variability, pharmacodynamic variability, and antimitogram performance. The model recapitulated tumor dynamics and standalone therapeutic drug monitoring efficacy consistent with published clinical results. Simulations showed that combining pharmacokinetic and pharmacodynamic optimization should increase PFS in a synergistic fashion. Simulated data were then used to compute required clinical trial sizes, which were 30% to 90% smaller when pharmacokinetic optimization was added to pharmacodynamic optimization. This improvement was observed even when pharmacokinetic optimization alone exhibited only modest benefit. Overall, our work illustrates the synergy derived from combining antimitograms with therapeutic drug monitoring, permitting a disproportionate reduction of the trial size required to prove a benefit on PFS. Accordingly, we suggest that strategies with benefits too small for standalone clinical trials could be validated in combination in a similar manner.Significance: This work offers a method to reduce the number of patients needed for a clinical trial to prove the hypothesized benefit of a drug to progression-free survival, possibly easing opportunities to evaluate combinations. Cancer Res; 78(7); 1873–82. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2Edlbqu
Pyruvate Dehydrogenase PDH-E1{beta} Controls Tumor Progression by Altering the Metabolic Status of Cancer Cells
Downregulation of pyruvate dehydrogenase (PDH) is critical for the aberrant preferential activation of glycolysis in cancer cells under normoxic conditions. Phosphorylation-dependent inhibition of PDH is a relevant event in this process, but it is not durable as it relies on PDH kinases that are activated ordinarily under hypoxic conditions. Thus, it remains unclear how PDH is durably downregulated in cancer cells that are not hypoxic. Building on evidence that PDH activity depends on the stability of a multi-protein PDH complex, we found that the PDH-E1β subunit of the PDH complex is downregulated to inhibit PDH activity under conditions of prolonged hypoxia. After restoration of normoxic conditions, reduced expression of PDH-E1β was sustained such that glycolysis remained highly activated. Notably, PDH-E1β silencing in cancer cells produced a metabolic state strongly resembling the Warburg effect, but inhibited tumor growth. Conversely, enforced exogenous expression of PDH-E1β durably increased PDH activity and promoted the malignant growth of breast cancer cells in vivo. Taken together, our results establish the specific mechanism through which PDH acts as an oncogenic factor by tuning glycolytic metabolism in cancer cells.Significance: This seminal study offers a mechanistic explanation for why glycolysis is aberrantly activated in normoxic cancer cells, offering insights into this long-standing hallmark of cancer termed the Warburg effect. Cancer Res; 78(7); 1592–603. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2J9zznv
Fc-Mediated Anomalous Biodistribution of Therapeutic Antibodies in Immunodeficient Mouse Models
A critical benchmark in the development of antibody-based therapeutics is demonstration of efficacy in preclinical mouse models of human disease, many of which rely on immunodeficient mice. However, relatively little is known about how the biology of various immunodeficient strains impacts the in vivo fate of these drugs. Here we used immunoPET radiotracers prepared from humanized, chimeric, and murine mAbs against four therapeutic oncologic targets to interrogate their biodistribution in four different strains of immunodeficient mice bearing lung, prostate, and ovarian cancer xenografts. The immunodeficiency status of the mouse host as well as both the biological origin and glycosylation of the antibody contributed significantly to the anomalous biodistribution of therapeutic monoclonal antibodies in an Fc receptor-dependent manner. These findings may have important implications for the preclinical evaluation of Fc-containing therapeutics and highlight a clear need for biodistribution studies in the early stages of antibody drug development.Significance: Fc/FcγR-mediated immunobiology of the experimental host is a key determinant to preclinical in vivo tumor targeting and efficacy of therapeutic antibodies. Cancer Res; 78(7); 1820–32. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2Ed29jS
Polyol Pathway Links Glucose Metabolism to the Aggressiveness of Cancer Cells
Cancer cells alter their metabolism to support their malignant properties. In this study, we report that the glucose-transforming polyol pathway (PP) gene aldo-keto-reductase-1-member-B1 (AKR1B1) strongly correlates with epithelial-to-mesenchymal transition (EMT). This association was confirmed in samples from lung cancer patients and from an EMT-driven colon cancer mouse model with p53 deletion. In vitro, mesenchymal-like cancer cells showed increased AKR1B1 levels, and AKR1B1 knockdown was sufficient to revert EMT. An equivalent level of EMT suppression was measured by targeting the downstream enzyme sorbitol-dehydrogenase (SORD), further pointing at the involvement of the PP. Comparative RNA sequencing confirmed a profound alteration of EMT in PP-deficient cells, revealing a strong repression of TGFβ signature genes. Excess glucose was found to promote EMT through autocrine TGFβ stimulation, while PP-deficient cells were refractory to glucose-induced EMT. These data show that PP represents a molecular link between glucose metabolism, cancer differentiation, and aggressiveness, and may serve as a novel therapeutic target.Significance: A glucose-transforming pathway in TGFβ-driven epithelial-to-mesenchymal transition provides novel mechanistic insights into the metabolic control of cancer differentiation. Cancer Res; 78(7); 1604–18. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2ImrAlP
STAT3/PIAS3 Levels Serve as “Early Signature” Genes in the Development of High-Grade Serous Carcinoma from the Fallopian Tube
The initial molecular events that lead to malignant transformation of the fimbria of the fallopian tube (FT) through high-grade serous ovarian carcinoma (HGSC) remain poorly understood. In this study, we report that increased expression of signal transducer and activator of transcription 3 (pSTAT3 Tyr705) and suppression or loss of protein inhibitor of activated STAT3 (PIAS3) in FT likely drive HGSC. We evaluated human tissues-benign normal FT, tubal-peritoneal junction (TPJ), p53 signature FT tissue, tubal intraepithelial lesion in transition (TILT), serous tubal intraepithelial carcinoma (STIC) without ovarian cancer, and HGSC for expression of STAT3/PIAS3 (compared with their known TP53 signature) and their target proliferation genes. We observed constitutive activation of STAT3 and low levels or loss of PIAS3 in the TPJ, p53 signature, TILT, and STIC through advanced stage IV (HGSC) tissues. Elevated expression of pSTAT3 Tyr705 and decreased levels of PIAS3 appeared as early as TPJ and the trend continued until very advanced stage HGSC (compared with high PIAS3 and low pSTAT3 expression in normal benign FT). Exogenous expression of STAT3 in FT cells mediated translocation of pSTAT3 and c-Myc into the nucleus. In vivo experiments demonstrated that overexpression of STAT3 in FT secretory epithelial cells promoted tumor progression and metastasis, mimicking the clinical disease observed in patients with HGSC. Thus, we conclude that the STAT3 pathway plays a role in the development and progression of HGSC from its earliest premalignant states.Significance: Concomitant gain of pSTAT3 Tyr705 and loss of PIAS3 appear critical for initiation and development of high-grade serous carcinoma. Cancer Res; 78(7); 1739–50. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2Imb311
Keratin 19 Expression in Hepatocellular Carcinoma Is Regulated by Fibroblast-Derived HGF via a MET-ERK1/2-AP1 and SP1 Axis
Keratin (KRT) 19 is a poor prognostic marker for hepatocellular carcinoma (HCC); however, regulatory mechanisms underlying its expression remain unclear. We have previously reported the presence of fibrous tumor stroma in KRT19-positive HCC, suggesting that cross-talk between cancer-associated fibroblasts (CAF) and tumor epithelial cells could regulate KRT19 expression. This was investigated in this study using an in vitro model of paracrine interaction between HCC cell lines (HepG2, SNU423) and hepatic stellate cells (HSC), a major source of hepatic myofibroblasts. HSCs upregulated transcription and translation of KRT19 in HCC cells via paracrine interactions. Mechanistically, hepatocyte growth factor (HGF) from HSCs activated c-MET and the MEK–ERK1/2 pathway, which upregulated KRT19 expression in HCC cells. Furthermore, AP1 (JUN/FOSL1) and SP1, downstream transcriptional activators of ERK1/2, activated KRT19 expression in HCC cells. In clinical specimens of human HCC (n = 339), HGF and KRT19 protein expression correlated with CAF levels. In addition, HGF or MET protein expression was associated with FOSL1 and KRT19 expression and was found to be a poor prognostic factor. Analysis of data from The Cancer Genome Atlas also revealed KRT19 expression was closely associated with CAF and MET-mediated signaling activities. These results provide insights into the molecular background of KRT19-positive HCC that display an aggressive phenotype.Significance: These findings reveal KRT19 expression in hepatocellular carcinoma is regulated by cross-talk between cancer-associated fibroblasts and HCC cells, illuminating new therapeutic targets for this aggressive disease. Cancer Res; 78(7); 1619–31. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GLAMCP
TRIM59 Promotes Gliomagenesis by Inhibiting TC45 Dephosphorylation of STAT3
Aberrant EGFR signaling is a common driver of glioblastoma (GBM) pathogenesis; however, the downstream effectors that sustain this oncogenic pathway remain unclarified. Here we demonstrate that tripartite motif-containing protein 59 (TRIM59) acts as a new downstream effector of EGFR signaling by regulating STAT3 activation in GBM. EGFR signaling led to TRIM59 upregulation through SOX9 and enhanced the interaction between TRIM59 and nuclear STAT3, which prevents STAT3 dephosphorylation by the nuclear form of T-cell protein tyrosine phosphatase (TC45), thereby maintaining transcriptional activation and promoting tumorigenesis. Silencing TRIM59 suppresses cell proliferation, migration, and orthotopic xenograft brain tumor formation of GBM cells and glioma stem cells. Evaluation of GBM patient samples revealed an association between EGFR activation, TRIM59 expression, STAT3 phosphorylation, and poor prognoses. Our study identifies TRIM59 as a new regulator of oncogenic EGFR/STAT3 signaling and as a potential therapeutic target for GBM patients with EGFR activation.Significance: These findings identify a novel component of the EGFR/STAT3 signaling axis in the regulation of glioma tumorigenesis. Cancer Res; 78(7); 1792–804. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2EdldyC
MBD2 Ablation Impairs Lymphopoiesis and Impedes Progression and Maintenance of T-ALL
Aberrant DNA methylation patterns in leukemia might be exploited for therapeutic targeting. In this study, we employed a genetically deficient mouse model to explore the role of the methylated DNA binding protein MBD2 in normal and malignant hematopoiesis. MBD2 ablation led to diminished lymphocytes. Functional defects of the lymphoid compartment were also observed after in vivo reconstitution of MBD2-deficient hematopoietic stem cells (HSC). In an established model of Notch1-driven T-cell acute lymphoblastic leukemia (T-ALL), MBD2 ablation impeded malignant progression and maintenance by attenuating the Wnt signaling pathway. In clinical specimens of human T-ALL, Wnt signaling pathway signatures were significantly enhanced and positively correlated with the expression and function of MBD2. Furthermore, a number of typical Wnt signaling inhibitory genes were abnormally hypermethylated in primary human T-ALL. Abnormal activation of Wnt signaling in T-ALL was switched off by MBD2 deletion, partially by reactivating epigenetically silenced Wnt signaling inhibitors. Taken together, our results define essential roles for MBD2 in lymphopoiesis and T-ALL and suggest MBD2 as a candidate therapeutic target in T-ALL.Significance: This study highlights a methylated DNA binding protein as a candidate therapeutic target to improve the treatment of T-cell acute lymphoblastic leukemias, as a new starting point for developing epigenetic therapy in this and other lymphoid malignancies. Cancer Res; 78(7); 1632–42. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2EfTtJS
Selective mTORC2 Inhibitor Therapeutically Blocks Breast Cancer Cell Growth and Survival
Small-molecule inhibitors of the mTORC2 kinase (torkinibs) have shown efficacy in early clinical trials. However, the torkinibs under study also inhibit the other mTOR-containing complex mTORC1. While mTORC1/mTORC2 combined inhibition may be beneficial in cancer cells, recent reports describe compensatory cell survival upon mTORC1 inhibition due to loss of negative feedback on PI3K, increased autophagy, and increased macropinocytosis. Genetic models suggest that selective mTORC2 inhibition would be effective in breast cancers, but the lack of selective small-molecule inhibitors of mTORC2 have precluded testing of this hypothesis to date. Here we report the engineering of a nanoparticle-based RNAi therapeutic that can effectively silence the mTORC2 obligate cofactor Rictor. Nanoparticle-based Rictor ablation in HER2-amplified breast tumors was achieved following intratumoral and intravenous delivery, decreasing Akt phosphorylation and increasing tumor cell killing. Selective mTORC2 inhibition in vivo, combined with the HER2 inhibitor lapatinib, decreased the growth of HER2-amplified breast cancers to a greater extent than either agent alone, suggesting that mTORC2 promotes lapatinib resistance, but is overcome by mTORC2 inhibition. Importantly, selective mTORC2 inhibition was effective in a triple-negative breast cancer (TNBC) model, decreasing Akt phosphorylation and tumor growth, consistent with our findings that RICTOR mRNA correlates with worse outcome in patients with basal-like TNBC. Together, our results offer preclinical validation of a novel RNAi delivery platform for therapeutic gene ablation in breast cancer, and they show that mTORC2-selective targeting is feasible and efficacious in this disease setting.Significance: This study describes a nanomedicine to effectively inhibit the growth regulatory kinase mTORC2 in a preclinical model of breast cancer, targeting an important pathogenic enzyme in that setting that has been undruggable to date. Cancer Res; 78(7); 1845–58. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2EdlbXw
Forkhead Box F2 Suppresses Gastric Cancer through a Novel FOXF2-IRF2BPL-{beta}-Catenin Signaling Axis
DNA methylation has been identified as a hallmark of gastric cancer (GC). Identifying genes that are repressed by DNA promoter methylation is essential in providing insights into the molecular pathogenesis of gastric cancer. Using genome-wide methylation studies, we identified that transcription factor forkhead box F2 (FOXF2) was preferentially methylated in gastric cancer. We then investigated the functional significance and clinical implication of FOXF2 in gastric cancer. FOXF2 was silenced in gastric cancer cell lines and cancer tissues by promoter methylation, which was negatively associated with mRNA expression. Ectopic expression of FOXF2 inhibited proliferation, colony formation, G1–S cell-cycle transition, induced apoptosis of gastric cancer cell lines, and suppressed growth of xenograft tumors in nude mice; knockdown of FOXF2 elicited opposing effects. FOXF2 inhibited Wnt signaling by inducing β-catenin protein ubiquitination and degradation independently of GSK-3β. FOXF2 directly bound the promoter of E3 ligase interferon regulatory factor 2-binding protein-like (IRF2BPL) and induced its transcriptional expression. IRF2BPL in turn interacted with β-catenin, increasing its ubiquitination and degradation. Multivariate Cox regression analysis identified FOXF2 hypermethylation as an independent prognostic factor of poor survival in early-stage gastric cancer patients. In conclusion, FOXF2 is a critical tumor suppressor in gastric carcinogenesis whose methylation status serves as an independent prognostic factor for gastric cancer patients.Significance: FOXF2-mediated upregulation of the E3 ligase IRF2BPL drives ubiquitylation and degradation of β-catenin in gastric cancer, blunting Wnt signaling and suppressing carcinogenesis. Cancer Res; 78(7); 1643–56. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GNOntx
Highlights from Recent Cancer Literature
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GNOq8H
CCR5 Governs DNA Damage Repair and Breast Cancer Stem Cell Expansion
The functional significance of the chemokine receptor CCR5 in human breast cancer epithelial cells is poorly understood. Here, we report that CCR5 expression in human breast cancer correlates with poor outcome. CCR5+ breast cancer epithelial cells formed mammospheres and initiated tumors with >60-fold greater efficiency in mice. Reintroduction of CCR5 expression into CCR5-negative breast cancer cells promoted tumor metastases and induced DNA repair gene expression and activity. CCR5 antagonists Maraviroc and Vicriviroc dramatically enhanced cell killing mediated by DNA-damaging chemotherapeutic agents. Single-cell analysis revealed CCR5 governs PI3K/Akt, ribosomal biogenesis, and cell survival signaling. As CCR5 augments DNA repair and is reexpressed selectively on cancerous, but not normal breast epithelial cells, CCR5 inhibitors may enhance the tumor-specific activities of DNA damage response–based treatments, allowing a dose reduction of standard chemotherapy and radiation.Significance: This study offers a preclinical rationale to reposition CCR5 inhibitors to improve the treatment of breast cancer, based on their ability to enhance the tumor-specific activities of DNA-damaging chemotherapies administered in that disease. Cancer Res; 78(7); 1657–71. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2EfTr4I
miR-508 Defines the Stem-like/Mesenchymal Subtype in Colorectal Cancer
Colorectal cancer includes an invasive stem-like/mesenchymal subtype, but its genetic drivers, functional, and clinical relevance are uncharacterized. Here we report the definition of an altered miRNA signature defining this subtype that includes a major genomic loss of miR-508. Mechanistic investigations showed that this miRNA affected the expression of cadherin CDH1 and the transcription factors ZEB1, SALL4, and BMI1. Loss of miR-508 in colorectal cancer was associated with upregulation of the novel hypoxia-induced long noncoding RNA AK000053. Ectopic expression of miR-508 in colorectal cancer cells blunted epithelial-to-mesenchymal transition (EMT), stemness, migration, and invasive capacity in vitro and in vivo. In clinical colorectal cancer specimens, expression of miR-508 negatively correlated with stemness and EMT-associated gene expression and positively correlated with patient survival. Overall, our results showed that miR-508 is a key functional determinant of the stem-like/mesenchymal colorectal cancer subtype and a candidate therapeutic target for its treatment.Significance: These results define a key functional determinant of a stem-like/mesenchymal subtype of colorectal cancers and a candidate therapeutic target for its treatment. Cancer Res; 78(7); 1751–65. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GM5BY9
Antiestrogen Therapy Increases Plasticity and Cancer Stemness of Prolactin-Induced ER{alpha}+ Mammary Carcinomas
Although antiestrogen therapies are successful in many patients with estrogen receptor alpha-positive (ERα+) breast cancer, 25% to 40% fail to respond. Although multiple mechanisms underlie evasion of these treatments, including tumor heterogeneity and drug-resistant cancer stem cells (CSC), further investigations have been limited by the paucity of preclinical ERα+ tumor models. Here, we examined a mouse model of prolactin-induced aggressive ERα+ breast cancer, which mimics the epidemiologic link between prolactin exposure and increased risk for metastatic ERα+ tumors. Like a subset of ERα+ patient cancers, the prolactin-induced adenocarcinomas contained two major tumor subpopulations that expressed markers of normal luminal and basal epithelial cells. CSC activity was distributed equally across these two tumor subpopulations. Treatment with the selective estrogen receptor downregulator (SERD), ICI 182,780 (ICI), did not slow tumor growth, but induced adaptive responses in CSC activity, increased markers of plasticity including target gene reporters of Wnt/Notch signaling and epithelial–mesenchymal transition, and increased double-positive (K8/K5) cells. In primary tumorsphere cultures, ICI stimulated CSC self-renewal and was able to overcome the dependence of self-renewal upon Wnt or Notch signaling individually, but not together. Our findings demonstrate that treatment of aggressive mixed lineage ERα+ breast cancers with a SERD does not inhibit growth, but rather evokes tumor cell plasticity and regenerative CSC activity, predicting likely negative impacts on patient tumors with these characteristics.Significance: This study suggests that treatment of a subset of ERα+ breast cancers with antiestrogen therapies may not only fail to slow growth but also promote aggressive behavior by evoking tumor cell plasticity and regenerative CSC activity. Cancer Res; 78(7); 1672–84. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2J9zyzX
Metformin-Induced Reduction of CD39 and CD73 Blocks Myeloid-Derived Suppressor Cell Activity in Patients with Ovarian Cancer
Metformin is a broadly prescribed drug for type 2 diabetes that exerts antitumor activity, yet the mechanisms underlying this activity remain unclear. We show here that metformin treatment blocks the suppressive function of myeloid-derived suppressor cells (MDSC) in patients with ovarian cancer by downregulating the expression and ectoenzymatic activity of CD39 and CD73 on monocytic and polymononuclear MDSC subsets. Metformin triggered activation of AMP-activated protein kinase α and subsequently suppressed hypoxia-inducible factor α, which was critical for induction of CD39/CD73 expression in MDSC. Furthermore, metformin treatment correlated with longer overall survival in diabetic patients with ovarian cancer, which was accompanied by a metformin-induced reduction in the frequency of circulating CD39+CD73+ MDSC and a concomitant increase in the antitumor activities of circulating CD8+ T cells. Our results highlight a direct effect of metformin on MDSC and suggest that metformin may yield clinical benefit through improvement of antitumor T-cell immunity by dampening CD39/CD73-dependent MDSC immunosuppression in ovarian cancer patients.Significance: The antitumor activity of an antidiabetes drug is attributable to reduced immunosuppressive activity of myeloid-derived tumor suppressor cells. Cancer Res; 78(7); 1779–91. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GLluxS
Downregulation of Membrane Trafficking Proteins and Lactate Conditioning Determine Loss of Dendritic Cell Function in Lung Cancer
Restoring antigen presentation for efficient and durable activation of tumor-specific CD8+ T-cell responses is pivotal to immunotherapy, yet the mechanisms that cause subversion of dendritic cell (DC) functions are not entirely understood, limiting the development of targeted approaches. In this study, we show that bona fide DCs resident in lung tumor tissues or DCs exposed to factors derived from whole lung tumors become refractory to endosomal and cytosolic sensor stimulation and fail to secrete IL12 and IFNI. Tumor-conditioned DC exhibited downregulation of the SNARE VAMP3, a regulator of endosomes trafficking critical for cross-presentation of tumor antigens and DC-mediated tumor rejection. Dissection of cell-extrinsic suppressive pathways identified lactic acid in the tumor microenvironment as sufficient to inhibit type-I IFN downstream of TLR3 and STING. DC conditioning by lactate also impacted adaptive function, accelerating antigen degradation and impairing cross-presentation. Importantly, DCs conditioned by lactate failed to prime antitumor responses in vivo. These findings provide a new mechanistic viewpoint to the concept of DC suppression and hold potential for future therapeutic approaches.Significance: These findings provide insight into the cell-intrinsic and cell-extrinsic mechanisms that cause loss of presentation of tumor-specific antigens in lung cancer tissues. Cancer Res; 78(7); 1685–99. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2EfTq0E
PHD3 Controls Lung Cancer Metastasis and Resistance to EGFR Inhibitors through TGF{alpha}
Lung cancer is the leading cause of cancer-related death worldwide, in large part due to its high propensity to metastasize and to develop therapy resistance. Adaptive responses to hypoxia and epithelial–mesenchymal transition (EMT) are linked to tumor metastasis and drug resistance, but little is known about how oxygen sensing and EMT intersect to control these hallmarks of cancer. Here, we show that the oxygen sensor PHD3 links hypoxic signaling and EMT regulation in the lung tumor microenvironment. PHD3 was repressed by signals that induce EMT and acted as a negative regulator of EMT, metastasis, and therapeutic resistance. PHD3 depletion in tumors, which can be caused by the EMT inducer TGFβ or by promoter methylation, enhanced EMT and spontaneous metastasis via HIF-dependent upregulation of the EGFR ligand TGFα. In turn, TGFα stimulated EGFR, which potentiated SMAD signaling, reinforcing EMT and metastasis. In clinical specimens of lung cancer, reduced PHD3 expression was linked to poor prognosis and to therapeutic resistance against EGFR inhibitors such as erlotinib. Reexpression of PHD3 in lung cancer cells suppressed EMT and metastasis and restored sensitivity to erlotinib. Taken together, our results establish a key function for PHD3 in metastasis and drug resistance and suggest opportunities to improve patient treatment by interfering with the feedforward signaling mechanisms activated by PHD3 silencing.Significance: This study links the oxygen sensor PHD3 to metastasis and drug resistance in cancer, with implications for therapeutic improvement by targeting this system. Cancer Res; 78(7); 1805–19. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2JaZriF
Tumor-Stroma IL1{beta}-IRAK4 Feedforward Circuitry Drives Tumor Fibrosis, Chemoresistance, and Poor Prognosis in Pancreatic Cancer
Targeting the desmoplastic stroma of pancreatic ductal adenocarcinoma (PDAC) holds promise to augment the effect of chemotherapy, but success in the clinic has thus far been limited. Preclinical mouse models suggest that near-depletion of cancer-associated fibroblasts (CAF) carries a risk of accelerating PDAC progression, underscoring the need to concurrently target key signaling mechanisms that drive the malignant attributes of both CAF and PDAC cells. We previously reported that inhibition of IL1 receptor–associated kinase 4 (IRAK4) suppresses NFκB activity and promotes response to chemotherapy in PDAC cells. In this study, we report that CAF in PDAC tumors robustly express activated IRAK4 and NFκB. IRAK4 expression in CAF promoted NFκB activity, drove tumor fibrosis, and supported PDAC cell proliferation, survival, and chemoresistance. Cytokine array analysis of CAF and microarray analysis of PDAC cells identified IL1β as a key cytokine that activated IRAK4 in CAF. Targeting IRAK4 or IL1β rendered PDAC tumors less fibrotic and more sensitive to gemcitabine. In clinical specimens of human PDAC, high stromal IL1β expression associated strongly with poor overall survival. Together, our studies establish a tumor–stroma IL1β-IRAK4 feedforward signal that can be therapeutically disrupted to increase chemotherapeutic efficacy in PDAC.Significance: Targeting the IL1β-IRAK4 signaling pathway potentiates the effect of chemotherapy in pancreatic cancer. Cancer Res; 78(7); 1700–12. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GNOm8X
A Next-Generation Chimeric Antigen Receptor Induces JAK-STAT Signaling [Immunotherapy]
CAR-T cells designed to activate JAK–STAT signaling show enhanced persistence and antitumor activity.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GqRfNp
Lutetium Lu 177 Dotatate Approved by FDA [News in Brief]
The FDA recently approved the radiopharmaceutical lutetium Lu 177 dotatate to treat patients with somatostatin receptor–positive gastroenteropancreatic neuroendocrine tumors. The approval was based on results of the phase III NETTER-1 trial.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pXKPdS
Noted [News in Brief]
A collection of recently published news items.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2JddEvq
NIH Offers Funding for Genome-Editing Projects [News in Brief]
The NIH is launching a new funding program for genome-editing technologies. The agency will award $190 million for projects including new delivery methods for genome editors, new editing technologies, and preclinical models to test their safety.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pYNWSR
Convergent Therapeutic Strategies to Overcome the Heterogeneity of Acquired Resistance in BRAFV600E Colorectal Cancer [Research Briefs]
Clonal heterogeneity associated with acquired resistance presents a critical therapeutic challenge. Whole-exome sequencing of paired tumor biopsies and targeted sequencing of cell-free DNA (cfDNA) from patients with BRAFV600E colorectal cancer receiving BRAF inhibitor combinations identified 14 distinct alterations in MAPK pathway components driving acquired resistance, with as many as eight alterations in a single patient. We developed a pooled clone system to study clonal outgrowth during acquired resistance, in vitro and in vivo. In vitro, the dynamics of individual resistant clones could be monitored in real time in cfDNA isolated from culture media during therapy. Outgrowth of multiple resistant clones was observed during therapy with BRAF, EGFR, and MEK inhibitor combinations. However, ERK inhibition, particularly in combination with BRAF and EGFR inhibition, markedly abrogated clonal outgrowth in vitro and in vivo. Thus, convergent, up-front therapy may suppress outgrowth of heterogeneous clones harboring clinically observed resistance alterations, which may improve clinical outcome.
Significance: We observed heterogeneous, recurrent alterations in the MAPK pathway as key drivers of acquired resistance in BRAFV600E colorectal cancer, with multiple concurrent resistance alterations detectable in individual patients. Using a novel pooled clone system, we identify convergent up-front therapeutic strategies capable of intercepting multiple resistance mechanisms as potential approaches to suppress emergence of acquired resistance. Cancer Discov; 8(4); 417–27. ©2018 AACR.
See related commentary by Janku, p. 389.
See related article by Corcoran et al., p. 428.
This article is highlighted in the In This Issue feature, p. 371
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2JdqrxR
Drug Combo Bests Sunitinib in RCC [News in Brief]
The phase III IMmotion151 trial found that the combination of atezolizumab and bevacizumab boosts progression-free survival compared with sunitinib in patients with advanced or metastatic renal cell carcinoma. The increase was 2.8 months in all patients and 3.5 months in patients with PD-L1–positive tumors.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pZdjDZ
The Oncolytic Adenovirus DNX-2401 Has Antitumor Activity in Glioblastoma [Clinical Trials]
In a phase I trial, DNX-2401 is safe and achieves durable responses in patients with recurrent glioma.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2Jd1Vgn
BMI Can Underestimate Breast Cancer Risk [News in Brief]
New findings suggest that postmenopausal women with high levels of body fat are at increased risk of ER-positive breast cancer, even if their body mass index is within the normal range. If confirmed, these results would put many more women at elevated risk for breast cancer than was previously thought, with implications for the way that healthy-weight women should be counseled to reduce their health risks.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pZ3i9E
Advances on the BRAF Front in Colorectal Cancer [In the Spotlight]
Summary: Colorectal cancer with BRAFV600E mutation can be effectively treated with combination approaches involving inhibition of BRAF, MEK, and EGFR proteins. However, activation of the MAPK pathway, often due to emergence of previously undetected molecular alterations, ultimately leads to adaptive therapeutic resistance. Novel combination strategies combining inhibition of BRAF, ERK, and EGFR can be used to prevent MAPK pathway–driven resistance and warrant further investigation. Cancer Discov; 8(4); 389–91. ©2018 AACR.
See related article by Corcoran et al., p. 428.
See related article by Hazar-Rethinam et al., p. 417.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2JbIK6N
Axitinib plus Pembrolizumab Is Effective in Renal Cell Carcinoma [Clinical Trials]
Axitinib plus pembrolizumab has a 73% response rate in previously untreated advanced renal cell carcinoma.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pY4UAF
MEF2C Phosphorylation Is Required for Chemotherapy Resistance in Acute Myeloid Leukemia [Research Articles]
In acute myeloid leukemia (AML), chemotherapy resistance remains prevalent and poorly understood. Using functional proteomics of patient AML specimens, we identified MEF2C S222 phosphorylation as a specific marker of primary chemoresistance. We found that Mef2cS222A/S222A knock-in mutant mice engineered to block MEF2C phosphorylation exhibited normal hematopoiesis, but were resistant to leukemogenesis induced by MLL–AF9. MEF2C phosphorylation was required for leukemia stem cell maintenance and induced by MARK kinases in cells. Treatment with the selective MARK/SIK inhibitor MRT199665 caused apoptosis and conferred chemosensitivity in MEF2C-activated human AML cell lines and primary patient specimens, but not those lacking MEF2C phosphorylation. These findings identify kinase-dependent dysregulation of transcription factor control as a determinant of therapy response in AML, with immediate potential for improved diagnosis and therapy for this disease.
Significance: Functional proteomics identifies phosphorylation of MEF2C in the majority of primary chemotherapy-resistant AML. Kinase-dependent dysregulation of this transcription factor confers susceptibility to MARK/SIK kinase inhibition in preclinical models, substantiating its clinical investigation for improved diagnosis and therapy of AML. Cancer Discov; 8(4); 478–97. ©2018 AACR.
This article is highlighted in the In This Issue feature, p. 371
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2JbfgWD
The PI3K{alpha} Inhibitor Alpelisib Has Activity in PIK3CA-altered Tumors [Clinical Trials]
The PI3Kα inhibitor alpelisib achieved a 58.2% disease control rate in PIK3CA-altered solid tumors.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pXQDnv
Asparagine Bioavailability Drives Breast Cancer Metastasis [Metastasis]
Asparagine depletion reduces breast cancer invasion and metastasis without affecting primary tumor growth.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2JbIJzL
AR Inhibition Achieves Responses in AR+ Triple-Negative Breast Cancer [Clinical Trials]
The AR inhibitor enzalutamide achieved responses in patients with advanced TNBC in a phase II trial.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pYwhdY
SETD1A Interacts with Cyclin K to Promote Leukemia Cell Survival [Leukemia]
SETD1A enhances leukemic cell growth and survival independent of its methyltransferase activity.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GqR6tl
SLFN11 Blocks DNA Replication Independently of ATR Activity [DNA Repair]
SLFN11 inhibits replication in response to DNA damage or cell cycle checkpoint inhibition.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pWuJB6
MEK Binding to KSR Promotes Allosteric Activation of BRAF [Structural Biology]
KSR–MEK complexes allosterically activate BRAF, which then phosphorylates a second MEK molecule.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GrTzUD
TGF{beta} Promotes Immune Evasion to Limit the Efficacy of Anti-PD-1/PD-L1 [Immunotherapy]
The TGFβ-activated stroma induces T-cell exclusion to suppress antitumor immunity.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pUvmLs
Tissue-Specific Immunoregulation: A Call for Better Understanding of the "Immunostat" in the Context of Cancer [Prospective]
Checkpoint inhibitor therapy has been a breakthrough in cancer research, but only some patients with cancer derive substantial benefit. Although mechanisms underlying sensitivity and resistance to checkpoint inhibitors are being elucidated, the importance of organ-specific regulation of immunity is currently underappreciated. Here, we call for a greater understanding of tissue-specific immunoregulation, namely, "tissue-specific immunostats," to make advances in treatments for cancer. A better understanding of how individual organs at baseline regulate the immune system could enable an improved precision medicine approach to cancer immunotherapy. Cancer Discov; 8(4); 395–402. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2J9FXuM
MYCN Amplification Promotes Enhancer Invasion in Neuroblastoma [Neuroblastoma]
Excess MYCN binds noncanonical enhancers as well as promoters to drive tumor-specific gene expression.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pYXAEW
Characterizing the Potency and Impact of Carbon Ion Therapy in a Primary Mouse Model of Soft Tissue Sarcoma
Carbon ion therapy (CIT) offers several potential advantages for treating cancers compared with X-ray and proton radiotherapy, including increased biological efficacy and more conformal dosimetry. However, CIT potency has not been characterized in primary tumor animal models. Here, we calculate the relative biological effectiveness (RBE) of carbon ions compared with X-rays in an autochthonous mouse model of soft tissue sarcoma. We used Cre/loxP technology to generate primary sarcomas in KrasLSL-G12D/+; p53fl/fl mice. Primary tumors were irradiated with a single fraction of carbon ions (10 Gy), X-rays (20 Gy, 25 Gy, or 30 Gy), or observed as controls. The RBE was calculated by determining the dose of X-rays that resulted in similar time to posttreatment tumor volume quintupling and exponential growth rate as 10 Gy carbon ions. The median tumor volume quintupling time and exponential growth rate of sarcomas treated with 10 Gy carbon ions and 30 Gy X-rays were similar: 27.3 and 28.1 days and 0.060 and 0.059 mm3/day, respectively. Tumors treated with lower doses of X-rays had faster regrowth. Thus, the RBE of carbon ions in this primary tumor model is 3. When isoeffective treatments of carbon ions and X-rays were compared, we observed significant differences in tumor growth kinetics, proliferative indices, and immune infiltrates. We found that carbon ions were three times as potent as X-rays in this aggressive tumor model and identified unanticipated differences in radiation response that may have clinical implications. Mol Cancer Ther; 17(4); 858–68. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GPqSQZ
Adaptive Resistance to Chemotherapy, A Multi-FAK-torial Linkage
Oncogenes provide tumor cells with a growth and survival advantage. Directed therapies targeted to oncogenic mutations (such as BRAF V600E) are part of effective late-stage melanoma treatment. However, tumors with BRAF V600E mutations, in approximately 10% of colorectal cancer, are generally treatment-insensitive. Research has identified various "feedback" mechanisms that result in BRAF signal pathway reactivation in response to BRAF inhibition. Herein, we highlight key findings from Chen and colleagues (this issue) showing that integrin-associated focal adhesion kinase (FAK) activation selectively occurs in BRAF V600E-mutant colorectal cancer cells in response to pharmacological BRAF inhibition. FAK activation results in elevated β-catenin protein levels, β-catenin nuclear localization, and increased gene transcription. Small-molecule inhibitors of β-catenin or FAK synergize with vemurafenib BRAF inhibitor to prevent BRAF V600E colorectal cancer cell proliferation in vitro and xenograft tumor growth in mice. This study complements findings linking FAK to β-catenin in intestinal tumorigenesis, resistance to radiotherapy, and cancer stem cell survival. Thus, FAK activation may occur as a frequent tumor cell "adaptive resistance" mechanism. Although FAK (PTK2) is not mutated in most cancers, targeting FAK activity in combinational approaches may limit tumor cell escape mechanisms and enhance durable responses to treatment. Mol Cancer Ther; 17(4); 719–23. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2ImfnNS
A Spatio-Temporal Model of Macrophage-Mediated Drug Resistance in Glioma Immunotherapy
The emergence of drug resistance is often an inevitable obstacle that limits the long-term effectiveness of clinical cancer chemotherapeutics. Although various forms of cancer cell-intrinsic mechanisms of drug resistance have been experimentally revealed, the role and the underlying mechanism of tumor microenvironment in driving the development of acquired drug resistance remain elusive, which significantly impedes effective clinical cancer treatment. Recent experimental studies have revealed a macrophage-mediated drug resistance mechanism in which the tumor microenvironment undergoes adaptation in response to macrophage-targeted colony-stimulating factor-1 receptor (CSF1R) inhibition therapy in gliomas. In this study, we developed a spatio-temporal model to quantitatively describe the interplay between glioma cells and CSF1R inhibitor–targeted macrophages through CSF1 and IGF1 pathways. Our model was used to investigate the evolutionary kinetics of the tumor regrowth and the associated dynamic adaptation of the tumor microenvironment in response to the CSF1R inhibitor treatment. The simulation result obtained using this model was in agreement with the experimental data. The sensitivity analysis revealed the key parameters involved in the model, and their potential impacts on the model behavior were examined. Moreover, we demonstrated that the drug resistance is dose-dependent. In addition, we quantitatively evaluated the effects of combined CSFR inhibition and IGF1 receptor (IGF1R) inhibition with the goal of designing more effective therapies for gliomas. Our study provides quantitative and mechanistic insights into the microenvironmental adaptation mechanisms that operate during macrophage-targeted immunotherapy and has implications for drug dose optimization and the design of more effective combination therapies. Mol Cancer Ther; 17(4); 814–24. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GGe1R1
Engaging Anaphase Catastrophe Mechanisms to Eradicate Aneuploid Cancers
Cancer cells often have supernumerary centrosomes that promote genomic instability, a pathognomonic feature of cancer. During mitosis, cancer cells with supernumerary centrosomes undergo bipolar cell division by clustering centrosomes into two poles. When supernumerary centrosome clustering is antagonized, cancer cells are forced to undergo multipolar division leading to death of daughter cells. This proapoptotic pathway, called anaphase catastrophe, preferentially eliminates aneuploid cancer cells and malignant tumors in engineered mouse models. Anaphase catastrophe occurs through the loss or inhibition of the centrosomal protein CP110, a direct cyclin-dependent kinase 1 (CDK1) and CDK2 target. Intriguingly, CP110 is repressed by the KRAS oncoprotein. This sensitizes KRAS-driven lung cancers (an unmet medical need) to respond to CDK2 inhibitors. Anaphase catastrophe-inducing agents like CDK1 and CDK2 antagonists are lethal to cancer cells with supernumerary centrosomes, but can relatively spare normal cells with two centrosomes. This mechanism is proposed to provide a therapeutic window in the cancer clinic following treatment with a CDK1 or CDK2 inhibitor. Taken together, anaphase catastrophe is a clinically tractable mechanism that promotes death of neoplastic tumors with aneuploidy, a hallmark of cancer. Mol Cancer Ther; 17(4); 724–31. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2Ime1mb
Characterization of ABBV-221, a Tumor-Selective EGFR-Targeting Antibody Drug Conjugate
Depatuxizumab mafodotin (depatux-m, ABT-414) is a tumor-selective antibody drug conjugate (ADC) comprised of the anti-EGFR antibody ABT-806 and the monomethyl auristatin F (MMAF) warhead. Depatux-m has demonstrated promising clinical activity in glioblastoma multiforme (GBM) patients and is currently being evaluated in clinical trials in first-line and recurrent GBM disease settings. Depatux-m responses have been restricted to patients with amplified EGFR, highlighting the need for therapies with activity against tumors with nonamplified EGFR overexpression. In addition, depatux-m dosing has been limited by corneal side effects common to MMAF conjugates. We hypothesized that a monomethyl auristatin E (MMAE) ADC utilizing an EGFR-targeting antibody with increased affinity may have broader utility against tumors with more modest EGFR overexpression while mitigating the risk of corneal side effects. We describe here preclinical characterization of ABBV-221, an EGFR-targeting ADC comprised of an affinity-matured ABT-806 conjugated to MMAE. ABBV-221 binds to a similar EGFR epitope as depatux-m and retains tumor selectivity with increased binding to EGFR-positive tumor cells and greater in vitro potency. ABBV-221 displays increased tumor uptake and antitumor activity against wild-type EGFR-positive xenografts with a greatly reduced incidence of corneal side effects relative to depatux-m. ABBV-221 has similar activity as depatux-m against an EGFR-amplified GBM patient derived xenograft (PDX) model and is highly effective alone and in combination with standard-of-care temozolomide in an EGFRvIII-positive GBM xenograft model. Based on these results, ABBV-221 has advanced to a phase I clinical trial in patients with advanced solid tumors associated with elevated levels of EGFR. Mol Cancer Ther; 17(4); 795–805. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2H2BObx
JAK2 Inhibitor SAR302503 Abrogates PD-L1 Expression and Targets Therapy-Resistant Non-small Cell Lung Cancers
Lung cancer is the leading cause of cancer-related deaths worldwide. Approximately 85% of all lung cancers are non–small cell histology [non–small cell lung cancer (NSCLC)]. Modern treatment strategies for NSCLC target driver oncogenes and immune checkpoints. However, less than 15% of patients survive beyond 5 years. Here, we investigated the effects of SAR302503 (SAR), a selective JAK2 inhibitor, on NSCLC cell lines and tumors. We show that SAR is cytotoxic to NSCLC cells, which exhibit resistance to genotoxic therapies, such as ionizing radiation, cisplatin, and etoposide. We demonstrate that constitutive IFN-stimulated gene expression, including an IFN-related DNA damage resistance signature, predicts for sensitivity to SAR. Importantly, tumor cell–intrinsic expression of PD-L1 is IFN-inducible and abrogated by SAR. Taken together, these findings suggest potential dual roles for JAK2 inhibitors, both as a novel monotherapy in NSCLCs resistant to genotoxic therapies, and in tandem with immune checkpoint inhibition. Mol Cancer Ther; 17(4); 732–9. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2ImtOSd
MCT4 Expression Is a Potential Therapeutic Target in Colorectal Cancer with Peritoneal Carcinomatosis
Monocarboxylate transporters (MCT) are transmembrane proteins that control the lactate metabolism and are associated with poor prognosis in solid tumors, including colorectal cancer. Here, we aimed to investigate the biological and clinical role of MCTs in colorectal cancer and to assess the potential of therapeutic application. A total of 16 human colorectal cancer cell lines, 11 patient-derived cells from malignant ascites [patient-derived cells (PDC)], and 39 matched pairs of primary colorectal cancer and normal colorectal tissues were used to assess the role of MCT in vitro and in vivo. siRNA methodology was used to determine the effect of MCT inhibition and molecular mechanism of hypoxia- and angiogenesis-related factors in addition to MCT4. The effect of MCT inhibition was confirmed in mouse xenograft models. MCT4 expression in surgical tissue was evaluated by IHC and used for survival analysis. Expression of MCTs was demonstrated in colorectal cancer cell lines. siRNA-mediated MCT silencing caused significant decline of cell proliferation both in vitro and in vivo. An additive effect of MCT inhibition was induced by combined treatment with chemotherapy or radiotherapy. In particular, the expression of MCT4 was markedly increased in PDCs, and MCT4 inhibition significantly decreased PDC proliferation. Hypoxia-inducible factor 1-α (HIF1α) was also highly expressed in PDCs, whereas HIF1α knockdown reduced MCT4 expression and of other angiogenesis-related mediators. The patients with high MCT4 expression by IHC showed shorter relapse-free survival compared with low expression. These findings suggest that MCT4 may represent a new therapeutic target for colorectal cancer with peritoneal carcinomatosis and serve as a prognostic indicator. Mol Cancer Ther; 17(4); 838–48. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2H33LzH
Pharmacological and Structural Characterizations of Naquotinib, a Novel Third-Generation EGFR Tyrosine Kinase Inhibitor, in EGFR-Mutated Non-Small Cell Lung Cancer
Multiple epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (EGFR-TKI) have been developed to effectively inhibit EGFR-derived signals in non–small cell lung cancer (NSCLC). In this study, we assessed the efficacy of EGFR-TKIs, including a novel third-generation inhibitor naquotinib (ASP8273), in clinically relevant EGFR mutations, including L858R, exon 19 deletion, L858R+T790M, exon 19 deletion+T790M with or without a C797S mutation, and several exon 20 insertion mutations. Using structural analyses, we also elucidated the mechanism of activation and sensitivity/resistance to EGFR-TKIs in EGFR exon 20 insertion mutations. The efficacy of naquotinib in cells with L858R, exon 19 deletion and exon 19 deletion+T790M was comparable with that of osimertinib. Interestingly, naquotinib was more potent than osimertinib for L858R+T790M. Additionally, naquotinib and osimertinib had comparable efficacy and a wide therapeutic window for cells with EGFR exon 20 insertions. Structural modeling partly elucidated the mechanism of activation and sensitivity/resistance to EGFR-TKIs in two EGFR exon 20 insertion mutants, A767_V769dupASV and Y764_V765insHH. In summary, we have characterized the efficacy of EGFR-TKIs for NSCLC using in vitro and structural analyses and suggested the mechanism of activation and resistance to EGFR-TKIs of EGFR exon 20 insertion mutations. Our findings should guide the selection of appropriate EGFR-TKIs for the treatment of NSCLC with EGFR mutations and help clarify the biology of EGFR exon 20 insertion mutations. Mol Cancer Ther; 17(4); 740–50. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2Edo9v0
Highlights of This Issue
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2H5bGfS
Preclinical Evaluation of SCC244 (Glumetinib), a Novel, Potent, and Highly Selective Inhibitor of c-Met in MET-dependent Cancer Models
Because the receptor tyrosine kinase c-Met plays a critical role in tumor growth, metastasis, tumor angiogenesis, and drug resistance, the c-Met axis represents an attractive therapeutic target. Herein, we report the first preclinical characterization of SCC244, a novel, potent, and highly selective inhibitor of c-Met kinase. SCC244 showed subnanomolar potency against c-Met kinase activity and high selectivity versus 312 other tested protein kinases, making it one of the most selective c-Met inhibitors described to date. Moreover, this inhibitor profoundly and specifically inhibits c-Met signal transduction and thereby suppresses the c-Met–dependent neoplastic phenotype of tumor and endothelial cells. In xenografts of human tumor cell lines or non–small cell lung cancer and hepatocellular carcinoma patient-derived tumor tissue driven by MET aberration, SCC244 administration exhibits robust antitumor activity at the well-tolerated doses. In addition, the in vivo antitumor activity of SCC244 involves the inhibition of c-Met downstream signaling via a mechanism of combined antiproliferation and antiangiogenic effects. The results of the current study provide a strong foundation for the clinical investigation of SCC244 in patients with tumors harboring c-Met pathway alterations. Mol Cancer Ther; 17(4); 751–62. ©2017 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2Ilomii
Wnt/{beta}-Catenin Pathway Activation Mediates Adaptive Resistance to BRAF Inhibition in Colorectal Cancer
One of the most encouraging developments in oncology has been the success of BRAF inhibitors in BRAF-mutant melanoma. However, in contrast to its striking efficacy in BRAF-mutant melanomas, BRAF inhibitor monotherapy is ineffective in BRAF-mutant colorectal cancer. Although many studies on BRAF inhibitor resistance in colorectal cancer have focused on mechanisms underlying the reactivation of the EGFR/RAS/RAF/MEK/ERK pathway, the current study focuses on identifying novel adaptive signaling mechanisms, a fresh angle on colorectal cancer resistance to BRAF inhibition. We found that treatment with BRAF inhibitors (both current and next-generation BRAF inhibitors) upregulated the Wnt/β-catenin pathway in BRAFV600E-mutant colorectal cancer cell lines through activating the cytoplasmic tyrosine kinase focal adhesion kinase (FAK). The results showed that FAK activation upon BRAF inhibitor treatment did not require EGFR or ERK1/2 activation, implying that BRAF inhibitor treatment-induced hyperactivation of Wnt signaling is "pathway reactivation"-independent. BRAF inhibition–induced Wnt pathway activation was further validated in preclinical models of BRAFV600E-mutant colorectal cancer, including cell line xenograft model and a patient-derived xenograft model. Combined inhibition of BRAF/Wnt pathways or BRAF/FAK pathways exerted strong synergistic antitumor effects in cell culture model and mouse xenograft model. Overall, the current study has identified activation of the Wnt/β-catenin pathway as a novel fundamental cause of colon cancer resistance to BRAF inhibition. Our results suggest that although complete vertical pathway blockade is pivotal for effective and durable control of BRAF-mutant colorectal cancer, cotargeting parallel adaptive signaling—the Wnt/β-catenin pathway—is also essential. Mol Cancer Ther; 17(4); 806–13. ©2017 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2H2vNvp
SKLB-23bb, A HDAC6-Selective Inhibitor, Exhibits Superior and Broad-Spectrum Antitumor Activity via Additionally Targeting Microtubules
Our previous study reported that SKLB-23bb, an orally bioavailable HDAC6-selective inhibitor, exhibited superior antitumor efficiency both in vitro and in vivo in comparison with ACY1215, a HDAC6-selective inhibitor recently in phase II clinical trial. This study focused on the mechanism related to the activity of SKLB-23bb. We discovered that despite having HDAC6-selective inhibition equal to ACY1215, SKLB-23bb showed cytotoxic effects against a panel of solid and hematologic tumor cell lines at the low submicromolar level. Interestingly, in contrast to the reported HDAC6-selective inhibitors, SKLB-23bb was more efficient against solid tumor cells. Utilizing HDAC6 stably knockout cell lines constructed by CRISPR–Cas9 gene editing, we illustrated that SKLB-23bb could remain cytotoxic independent of HDAC6 status. Investigation of the mechanism confirmed that SKLB-23bb exerted its cytotoxic activity by additionally targeting microtubules. SKLB-23bb could bind to the colchicine site in β-tubulin and act as a microtubule polymerization inhibitor. Consistent with its microtubule-disrupting ability, SKLB-23bb also blocked tumor cell cycle at G2–M phase and triggered cellular apoptosis. In solid tumor xenografts, oral administration of SKLB-23bb efficiently inhibited tumor growth. These results suggested that SKLB-23bb was an orally bioavailable HDAC6 and microtubule dual targeting agent. The microtubule targeting profile enhanced the antitumor activity and expanded the antitumor spectrum of SKLB-23bb, thus breaking through the limitation of HDAC6 inhibitors. Mol Cancer Ther; 17(4); 763–75. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2Edo5vo
Essential Role of Polo-like Kinase 1 (Plk1) Oncogene in Tumor Growth and Metastasis of Tamoxifen-Resistant Breast Cancer
The most common therapy for estrogen receptor–positive breast cancer is antihormone therapy, such as tamoxifen. However, acquisition of resistance to tamoxifen in one third of patients presents a serious clinical problem. Polo-like kinase 1 (Plk1) is a key oncogenic regulator of completion of G2–M phase of the cell cycle. We assessed Plk1 expression in five chemoresistant cancer cell types and found that Plk1 and its downstream phosphatase Cdc25c were selectively overexpressed in tamoxifen-resistant MCF-7 (TAMR-MCF-7) breast cancer cells. Real-time monitoring of cell proliferation also showed that TAMR-MCF-7 cells were more sensitive to inhibition of cell proliferation by the ATP-competitive Plk1 inhibitor BI2536 than were the parent MCF-7 cells. Moreover, BI2536 suppressed expression of epithelial–mesenchymal transition marker proteins and 3D spheroid formation in TAMR-MCF-7 cells. Using TAMR-MCF-7 cell–implanted xenograft and spleen–liver metastasis models, we showed that BI2536 inhibited tumor growth and metastasis in vivo. Our results suggest that Plk1 could be a novel target for the treatment of tamoxifen-resistant breast cancer. Mol Cancer Ther; 17(4); 825–37. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2H1C6iJ
Relative Target Affinities of T-Cell-Dependent Bispecific Antibodies Determine Biodistribution in a Solid Tumor Mouse Model
Anti-HER2/CD3, a T-cell–dependent bispecific antibody (TDB) construct, induces T-cell–mediated cell death in cancer cells expressing HER2 by cross-linking tumor HER2 with CD3 on cytotoxic T cells, thereby creating a functional cytolytic synapse. TDB design is a very challenging process that requires consideration of multiple parameters. Although therapeutic antibody design strategy is commonly driven by striving for the highest attainable antigen-binding affinity, little is known about how the affinity of each TDB arm can affect the targeting ability of the other arm and the consequent distribution and efficacy. To our knowledge, no distribution studies have been published using preclinical models wherein the T-cell–targeting arm of the TDB is actively bound to T cells. We used a combined approach involving radiochemistry, invasive biodistribution, and noninvasive single-photon emission tomographic (SPECT) imaging to measure TDB distribution and catabolism in transgenic mice with human CD3 expression on T cells. Using CD3 affinity variants, we assessed the impact of CD3 affinity on short-term pharmacokinetics, tissue distribution, and cellular uptake. Our experimental approach determined the relative effects of (i) CD3 targeting to normal tissues, (ii) HER2 targeting to HER2-expressing tumors, and (iii) relative HER2/CD3 affinity, all as critical drivers for TDB distribution. We observed a strong correlation between CD3 affinity and distribution to T-cell–rich tissues, with higher CD3 affinity reducing systemic exposure and shifting TDB distribution away from tumor to T-cell–containing tissues. These observations have important implications for clinical translation of bispecific antibodies for cancer immunotherapy. Mol Cancer Ther; 17(4); 776–85. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2InR421
PIM Kinases Are a Potential Prognostic Biomarker and Therapeutic Target in Neuroblastoma
The majority of high-risk neuroblastoma patients are refractory to, or relapse on, current treatment regimens, resulting in 5-year survival rates of less than 50%. This emphasizes the urgent need to identify novel therapeutic targets. Here, we report that high PIM kinase expression is correlated with poor overall survival. Treatment of neuroblastoma cell lines with the pan-PIM inhibitors AZD1208 or PIM-447 suppressed proliferation through inhibition of mTOR signaling. In a panel of neuroblastoma cell lines, we observed a marked binary response to PIM inhibition, suggesting that specific genetic lesions control responses to PIM inhibition. Using a genome-wide CRISPR-Cas9 genetic screen, we identified NF1 loss as the major resistance mechanism to PIM kinase inhibitors. Treatment with AZD1208 impaired the growth of NF1 wild-type xenografts, while NF1 knockout cells were insensitive. Thus, our data indicate that PIM inhibition may be a novel targeted therapy in NF1 wild-type neuroblastoma. Mol Cancer Ther; 17(4); 849–57. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GOGcNE
MI130004, a Novel Antibody-Drug Conjugate Combining Trastuzumab with a Molecule of Marine Origin, Shows Outstanding In Vivo Activity against HER2-Expressing Tumors
In the search for novel payloads to design new antibody–drug conjugates (ADC), marine compounds represent an interesting opportunity given their unique chemical features. PM050489 is a marine compound that binds β-tubulin at a new site and disrupts the microtubule network, hence leading to mitotic aberrations and cell death. PM050489 has been conjugated to trastuzumab via Cys residues through a noncleavable linker, and the resulting ADC, named MI130004, has been studied. Analysis of MI130004 delivered data consistent with the presence of two molecules of PM050489 per antibody molecule, likely bound to both sides of the intermolecular disulfide bond connecting the antibody light and heavy chains. The antitumor activity of MI130004 was analyzed in vitro and in vivo in different cell lines of diverse tumor origin (breast, ovary, and gastric cancer) expressing different levels of HER2. MI130004 showed very high in vitro potency and good selectivity for tumor cells that overexpressed HER2. At the cellular level, MI130004 impaired tubulin polymerization, causing disorganization and disintegration of the microtubule network, which ultimately led to mitotic failure, mirroring the effect of its payload. Treatment with MI130004 in mice carrying histologically diverse tumors expressing HER2 induced a long-lasting antitumor effect with statistically significant inhibition of tumor growth coupled with increases in median survival time compared with vehicle or trastuzumab. These results strongly suggest that MI130004 is endowed with remarkable anticancer activity and confirm the extraordinary potential of marine compounds for the design of new ADCs. Mol Cancer Ther; 17(4); 786–94. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2EcXEG3
Impact of Chemical-Induced Mutational Load Increase on Immune Checkpoint Therapy in Poorly Responsive Murine Tumors
A recurring historic finding in cancer drug development is encouraging antitumor effects observed in tumor-bearing mice that fail to translate into the clinic. An intriguing exception to this pattern is immune checkpoint therapy, as the sustained tumor regressions observed in subsets of cancer patients are rare in mice. Reasoning that this may be due in part to relatively low mutational loads of mouse tumors, we mutagenized transplantable mouse tumor cell lines EMT-6/P, B16F1, RENCA, CT26, and MC38 in vitro with methylnitro-nitrosoguanidine (MNNG) or ethylmethane sulfonate (EMS) and tested their responsiveness to PD-L1 blockade. Exome sequencing confirmed an increase in somatic mutations by mutagen treatment, an effect mimicked in EMT-6 variants chronically exposed in vivo to cisplatin or cyclophosphamide. Certain mutagenized variants of B16F1, EMT-6/P, CT26, and MC38 (but not RENCA) were more immunogenic than their parents, yet anti-PD-L1 sensitization developed only in some EMT-6/P and B16F1 variants. Treatment response patterns corresponded with changes in immune cell infiltration and especially increases in CD8+ T cells. Chronically cisplatin-exposed EMT-6 variants were also more responsive to anti-PD-L1 therapy. Although tumor PD-L1 expression was upregulated in in vivo chemotherapy-exposed variants, PD-L1 expression levels were not consistently associated with anti-PD-L1 treatment activity across mutagenized or chemotherapy-exposed variants. In summary, mutagenized and more immunogenic mouse tumors were not universally sensitized to PD-L1 blockade. Chemically mutagenized variants may be useful to evaluate the impact of immunologically "hot" or "cold" tumors with a high mutational load, to which certain chemotherapy agents may contribute, on immunotherapy outcomes. Mol Cancer Ther; 17(4); 869–82. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GM8Z5j
Genome-Wide Gene Expression Changes in the Normal-Appearing Airway during the Evolution of Smoking-Associated Lung Adenocarcinoma
Smoking perpetuates in cytologically normal airways a molecular "field of injury" that is pertinent to lung cancer and early detection. The evolution of airway field changes prior to lung oncogenesis is poorly understood largely due to the long latency of lung cancer in smokers. Here, we studied airway expression changes prior to lung cancer onset in mice with knockout of the Gprc5a gene (Gprc5a–/–) and tobacco carcinogen (NNK) exposure and that develop the most common type of lung cancer, lung adenocarcinoma, within 6 months following exposure. Airway epithelial brushings were collected from Gprc5a–/– mice before exposure and at multiple times post-NNK until time of lung adenocarcinoma development and then analyzed by RNA sequencing. Temporal airway profiles were identified by linear models and analyzed by comparative genomics in normal airways of human smokers with and without lung cancer. We identified significantly altered profiles (n = 926) in the NNK-exposed mouse normal airways relative to baseline epithelia, a subset of which were concordantly modulated with smoking status in the human airway. Among airway profiles that were significantly modulated following NNK, we found that expression changes (n = 22) occurring as early as 2 months following exposure were significantly associated with lung cancer status when examined in airways of human smokers. Furthermore, a subset of a recently reported human bronchial gene classifier (Percepta; n = 56) was enriched in the temporal mouse airway profiles. We underscore evolutionarily conserved profiles in the normal-appearing airway that develop prior to lung oncogenesis and that comprise viable markers for early lung cancer detection in suspect smokers. Cancer Prev Res; 11(4); 237–48. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2Grsktk
Immunomodulatory Effects of Momordica charantia Extract in the Prevention of Oral Cancer
In recent times, bitter melon extract (BME) has gained significant attention for its anticancer efficacy against various malignancies. In this issue, Sur and colleagues show that BME prevents the development of 4-nitronitroquinoline 1-oxide–induced oral dysplasia and squamous cell carcinoma (SCC) in an immunocompetent mouse model. Importantly, gene ontology and pathway analyses revealed an elevated expression of s100a9, IL23a, IL1β, and PDCD1/PD1 of immune system during oral cancer development, which was significantly suppressed by BME. Overall, this study demonstrates the potential clinical benefits of BME in preventing and delaying the progression of oral dysplasia to SCC. Cancer Prev Res; 11(4); 185–6. ©2018 AACR.
See related article by Sur et al., p. 191
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pYIWh3
Pioglitazone Inhibits Periprostatic White Adipose Tissue Inflammation in Obese Mice
Obesity is associated with an increased incidence of high-grade prostate cancer and poor prognosis for prostate cancer patients. Recently, we showed that obesity-related periprostatic white adipose tissue (WAT) inflammation, characterized by crown-like structures (CLS) consisting of dead or dying adipocytes surrounded by macrophages, was associated with high-grade prostate cancer. It is possible, therefore, that agents that suppress periprostatic WAT inflammation will alter the development or progression of prostate cancer. Pioglitazone, a ligand of PPAR, is used to treat diabetes and possesses anti-inflammatory properties. Here, our main objectives were to determine whether pioglitazone inhibited obesity-related periprostatic WAT inflammation in mice and then to elucidate the underlying mechanism. Treatment with pioglitazone reduced the density of CLS in periprostatic fat and suppressed levels of TNFα, TGFβ, and the chemokine monocyte chemoattractant protein-1 (MCP-1). Importantly, the ability of pioglitazone to suppress periprostatic WAT inflammation was abrogated in MCP-1 knockout mice. Pioglitazone caused dose-dependent induction of both adiponectin, an anti-inflammatory adipokine, and its receptor AdipoR2 in cultured 3T3-L1 cells and in periprostatic WAT of obese mice. Pioglitazone blocked TNFα-mediated induction of MCP-1 in 3T3-L1 cells, an effect that was attenuated when either adiponectin or AdipoR2 were silenced. Taken together, pioglitazone-mediated induction of adiponectin suppressed the elevation in MCP-1 levels, thereby attenuating obesity-related periprostatic WAT inflammation. These findings strengthen the rationale for future efforts to determine whether targeting the PPAR–adiponectin–MCP-1 axis will decrease periprostatic adipose inflammation and thereby reduce the risk of high-grade prostate cancer or improve outcomes for men with prostate cancer. Cancer Prev Res; 11(4); 215–26. ©2017 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2Jahtle
The Conundrum of Omega-3 Fatty Acids in Cancer Prevention Studies: Which One? How Much? What Biomarkers?
Marine omega-3 fatty acids promote resolution of inflammation and have potential to reduce risk of obesity-related breast cancer. For prevention trials in obese women, inflammatory cytokines, aromatase, and measures of breast immune cell infiltration are logical, as are biomarkers of growth factor, adipokine, and estrogen signaling. Where best to look for marker change: in the circulation (easiest), in benign breast tissue (most relevant), or in visceral adipose (inflammation often most marked)? A null biomarker modulation trial may reflect limitations in design, source and dose of fatty acids, or biomarkers and should not lead to premature abandonment of marine omega-3 fatty acids for cancer prevention. Cancer Prev Res; 11(4); 187–90. ©2018 AACR.
See related article by Gucalp et al., p. 203
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pZgVWp
Accelerating the Pace of Cancer Prevention- Right Now
As a nation, we underinvest in prevention and fail to implement strategies that ensure all population groups equitably share in the return on investment in prevention research and the benefits of prevention effectiveness. There is significant evidence indicating that by applying knowledge that we already have to reduce tobacco, inactivity, and obesity (known modifiable causes of cancer), we can prevent more than 50% of cancers. Vaccination against HPV, aspirin and selective estrogen receptor modulators, and screening programs further reduce risk. Evidence-based prevention strategies are inconsistently implemented across the United States. Substantial variation across States indicates that there is much room for improvement in implementation of prevention. Implementation science applies innovative approaches to identifying, understanding, and developing strategies for overcoming barriers to the adoption, adaptation, integration, scale-up, and sustainability of evidence-based interventions, tools, policies, and guidelines that will prevent cancer through application of evidence-based interventions. When we get implementation of prevention programs right and at scale, we achieve substantial population benefits. Although many efforts are underway to maximize our knowledge about the causes and treatments of cancer, we can achieve reductions in the cancer burden right now by doing what we already know. The time to start is now. Cancer Prev Res; 11(4); 171–84. ©2018 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2J7ihax
Bitter Melon Prevents the Development of 4-NQO-Induced Oral Squamous Cell Carcinoma in an Immunocompetent Mouse Model by Modulating Immune Signaling
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide, and tobacco is one of the most common factors for HNSCC of the oral cavity. We have previously observed that bitter melon (Momordica charantia) extract (BME) exerts antiproliferative activity against several cancers including HNSCC. In this study, we investigated the preventive role of BME in 4-nitroquinoline 1-oxide (4-NQO) carcinogen-induced HNSCC. We observed that BME feeding significantly reduced the incidence of 4-NQO–induced oral cancer in a mouse model. Histologic analysis suggested control 4-NQO–treated mouse tongues showed neoplastic changes ranging from moderate dysplasia to invasive squamous cell carcinoma, whereas no significant dysplasia was observed in the BME-fed mouse tongues. We also examined the global transcriptome changes in normal versus carcinogen-induced tongue cancer tissues, and following BME feeding. Gene ontology and pathway analyses revealed a signature of biological processes including "immune system process" that is significantly dysregulated in 4-NQO–induced oral cancer. We identified elevated expression of proinflammatory genes, s100a9, IL23a, IL1β and immune checkpoint gene PDCD1/PD1, during oral cancer development. Interestingly, BME treatment significantly reduced their expression. Enhancement of MMP9 ("ossification" pathway) was noted during carcinogenesis, which was reduced in BME-fed mouse tongue tissues. Our study demonstrates the preventive effect of BME in 4-NQO–induced carcinogenesis. Identification of pathways involved in carcinogen-induced oral cancer provides useful information for prevention strategies. Together, our data strongly suggest the potential clinical benefits of BME as a chemopreventive agent in the control or delay of carcinogen-induced HNSCC development and progression. Cancer Prev Res; 11(4); 191–202. ©2017 AACR.
See related editorial by Rao, p. 185
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pYTw7E
Adiposity, Inflammation, and Breast Cancer Pathogenesis in Asian Women
Obesity is associated with white adipose tissue (WAT) inflammation in the breast, elevated levels of the estrogen biosynthetic enzyme, aromatase, and systemic changes that predispose to breast cancer development. We examined whether WAT inflammation and its associated systemic effects correlate with body fat levels in an Asian population where body mass index (BMI) is not an accurate assessment of obesity and cancer risk. We also investigated whether biologic differences could account for the greater proportion of premenopausal estrogen receptor (ER)–positive breast cancer in Asian versus Western countries. Breast WAT and fasting blood were prospectively collected from Taiwanese women undergoing mastectomy for breast cancer treatment. Body composition was measured in a subgroup using bioelectrical impedance analysis. WAT inflammation was defined by the presence of crown-like structures of the breast, which are composed of dead or dying adipocytes surrounded by macrophages. Findings were compared with U.S. Caucasian women. In the Taiwanese cohort (n = 72), breast WAT inflammation was present in 31 (43%) women and was associated with elevated BMI (P < 0.01) and increased levels of body fat (P < 0.01), C-reactive protein (P = 0.02), triglycerides (P < 0.01), insulin resistance scores (P = 0.04), and lower HDL cholesterol (P < 0.01). ER+ tumors were associated with greater body fat versus other subtypes (P = 0.03). Compared with U.S. Caucasians (n = 267), Taiwanese women had larger breast adipocytes despite lower BMI after adjusting for BMI and menopausal status (P = 0.01). A subclinical inflammatory state associated with increased adiposity and metabolic dysfunction could contribute to breast cancer pathogenesis in Asian women. Cancer Prev Res; 11(4); 227–36. ©2017 AACR.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2JewmTE
A Randomized Multicenter Phase II Study of Docosahexaenoic Acid in Patients with a History of Breast Cancer, Premalignant Lesions, or Benign Breast Disease
Obesity, a cause of subclinical inflammation, is a risk factor for the development of postmenopausal breast cancer and is associated with poorer cancer outcomes. Docosahexaenoic acid (DHA), an omega-3 fatty acid, possesses anti-inflammatory properties. We hypothesized that treatment with DHA would reduce the expression of proinflammatory genes and aromatase, the rate-limiting enzyme for estrogen biosynthesis, in benign breast tissue of overweight/obese women. A randomized, placebo-controlled, double-blind phase II study of DHA given for 12 weeks to overweight/obese women with a history of stage I–III breast cancer, DCIS/LCIS, Paget's disease, or proliferative benign breast disease was carried out. In this placebo controlled trial, the primary objective was to determine whether DHA (1,000 mg by mouth twice daily) reduced breast tissue levels of TNFα. Secondary objectives included evaluation of the effect of DHA on breast tissue levels of COX-2, IL1β, aromatase, white adipose tissue inflammation, and gene expression by RNA-seq. Red blood cell fatty acid levels were measured to assess compliance. From July 2013 to November 2015, 64 participants were randomized and treated on trial (32 women per arm). Increased levels of omega-3 fatty acids in red blood cells were detected following treatment with DHA (P < 0.001) but not placebo. Treatment with DHA did not alter levels of TNFα (P = 0.71), or other biomarkers including the transcriptome in breast samples. Treatment with DHA was overall well-tolerated. Although compliance was confirmed, we did not observe changes in the levels of prespecified biomarkers in the breast after treatment with DHA when compared with placebo. Cancer Prev Res; 11(4); 203–14. ©2018 AACR.
See related editorial by Fabian and Kimler, p. 187
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pYTvR8
Correction: Whole-Genome Sequencing of Salivary Gland Adenoid Cystic Carcinoma
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2Jahk1a
Oral health and chemotherapy act as cofactors in malnutrition in the elderly with other cancers than head and neck malignancies
Abstract
Objectives
This study explores whether the chemotherapy regimen has a role in inducing oral health problems and malnutrition in elderly patients with other cancers than head and neck malignancies.
Material and methods
An observational cross-sectional study was designed to compare the relationships between oral health and nutritional status between four groups of elderly differing in cancer or chemotherapy regimen. Data were collected using a questionnaire including the Mini-Nutritional Assessment (MNA), the Geriatric Oral Health Assessment Index (GOHAI) and questions about perception of xerostomia. The oral examinations recorded the number of functional dental units (PFU) and the presence of oral lesions.
Results
The sample comprised 46 patients receiving chemotherapy, 48 patients on a non-chemotherapy regimen, 45 persons in complete remission not under treatment and 53 non-cancer patients. Oral health perception was significantly worse in chemotherapy patients. They reported limiting the kinds of food they consumed, the discomfort felt when eating and took medications for oral pain. Oral lesions were frequent in chemotherapy participants. Nutritional status was related to the cancer treatment regimen (OR = 4.17; p value = 0.017), the presence of oral lesions (OR = 4.51; p value = 0.003), perception of xerostomia (OR = 3.54; p value = 0.012), the number of PFU (OR = 2.51; p value = 0.046) and GOHAI score (OR = 1.617; p value = 0.019).
Conclusion
The presence of oral lesions and the chemotherapeutic regimen were highly associated with nutritional status in older patients with cancer.
Clinical relevance
Dental professionals should be asked to intervene preventively and per-therapy to optimise oral health status in elderly patients with other cancers than head and neck malignancies.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2H4DpxB
Limited incision harvest of the rectus abdominis muscle flap
Abstract
Background
Rectus abdominis muscle harvest typically uses a long and continuous paramedian approach. To limit donor site morbidity, the senior author performs a limited incision approach particularly useful in medically vulnerable patients, including patients with sternal wound infections.
Methods
All patients of a single surgeon from 2000 to 2014 who underwent rectus harvest by one or two transverse incisions and use of lighted retractor were identified. Patients were categorized by indication, for "sternal wound coverage" or "non-sternal wound coverage." Co-morbidities, operative notes, and post-operative courses were evaluated. Comparisons were made to patients undergoing harvest by paramedian approach.
Results
Seventeen patients with a mean age of 61 underwent limited-incision rectus harvest. Nine patients had indication for "sternal wound coverage." Three patients had single transverse incision and six patients had double transverse incisions. One patient expired post-reconstruction day 3. One patient had complete abdominal and partial sternal wound dehiscence. No other donor site complications were observed. Eight patients had indication for "non-sternal wound coverage," including seven patients requiring free rectus for lower extremity defects and one a pedicled rectus abominis for pelvic osteomyelitis. No post-operative complications were observed in these non-sternal wound coverage patients. There was a trend toward improved wound healing and hospitalization time using the transverse compared to paramedian technique, although this was not significant.
Conclusions
The morbidity of the traditional paramedian incision for rectus harvest may be avoided using a limited skin incision approach. This is useful in patients with attenuated healing capacity and offers a lower risk approach to a traditionally risky donor site.
Level of Evidence: Level IV, therapeutic study.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GMiLEA
Oropharyngeal Dysphagia Evaluation Tools in Adults with Solid Malignancies Outside the Head and Neck and Upper GI Tract: A Systematic Review
Abstract
Dysphagia is often associated with head and neck and upper gastrointestinal (GI) tract cancers. Evidence suggests that those with solid malignancies in other primary sites may also have swallowing difficulties. Timely and accurate identification of dysphagia is important given the impact it has on hydration, medical treatment, nutrition, prognosis, and quality of life. A systematic review was conducted to identify swallow screening, evaluation, and quality of life tools for those with solid malignancies outside the head and neck and upper GI tract. Ten electronic databases, one journal and two published conference proceedings were searched. Following deduplication, 7435 studies were examined for relevance. No tools were validated solely in this cancer population, though some included this group in larger cohorts. Comments are provided on the diagnostic properties and applicability of these tools. In the absence of appropriate diagnostic instruments, the exact prevalence of dysphagia and its impact on clinical and psychosocial well-being remain unknown. Accurate and adequate measurement of therapeutic intervention is also compromised. This review establishes the need for validated dysphagia evaluation tools for this clinical population.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pXCNBK
Lymph node cancer of the mediastinum with a putative necrotic primary lesion in the lung: a case report
Abstract
Background
Although mediastinal lymph node cancer is presumed to originate in the lung, the primary site is usually unidentified, so the pathological course remains unclear. We recently encountered a case of mediastinal lymph node cancer having a putative primary lesion remaining in the lung as a necrotic focus.
Case presentation
The patient was a 56-year-old man who visited our department because computed tomography screening had revealed a nodular shadow in the lingular segment. However, on positron emission tomography, fluorine-18 deoxyglucose accumulation was detected in a subcarinal lymph node and not in the nodule in the lingular segment. Biopsy of the lung tumor and the lymph node was performed via minimal thoracotomy. Intraoperative pathologic examination showed necrosis alone and no malignant findings in the lung tumor. By contrast, carcinoma was detected in the lymph node. Additional subcarinal lymph node dissection was performed. Results of postoperative histopathologic examination indicated poorly differentiated adenocarcinoma of the subcarinal lymph node. Meanwhile, the nodule in the lingular segment was speculated to be a spontaneously resolved primary focus of lung cancer.
Conclusions
In this case, the primary lung cancer focus resolved spontaneously after lymph node metastasis, explaining the pathogenesis underlying mediastinal lymph node cancer of unknown primary site. For similar cases of malignancy, aggressive treatment, including surgery, is effective.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2Jacili
Guest Post – Down with STEMI – The OMI Manifesto by Pendell Meyers

Down with STEMI
EMCrit Project by Pendell Meyers.
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pXUfWr
RE: “Deployment And Preterm Birth Among US Army Soldiers”
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2GLBHn2
2017 Articles of the Year, Reviewers of the Year, and Figure of the Year
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2IkD9tw
How to cope with food allergy symptoms?
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2Ii5Szh
Oral food challenge using different target doses and time intervals between doses
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2H2ry2Q
Alternative Choices for Anterolateral Thigh Flaps Lacking Suitable Perforators: A Systematic Review

J reconstr Microsurg
DOI: 10.1055/s-0038-1639366
Background The anterolateral thigh (ALT) flap has become a predominant option in the field of reconstruction. However, some difficulties in harvesting flap exist due to the anatomical variability of the perforators. Reports have provided solutions for unreliable perforators. Although numerous cases that showed successful conversion to tensor fasciae latae (TFL) flap or anteromedial thigh (AMT) flap have been reported in the literature, none fully addresses the reliability of the perforators that have been described to date. Therefore, we conducted a systematic literature review to compare the reliability of the TFL flap with that of the AMT flap when an ALT flap perforator is not suitable. Methods A systematic review of the MEDLINE, PubMed, and Cochrane Library electronic databases was performed to compare the characteristics of TFL and AMT flap perforators. Results A total of 13 articles were included for review. The mean number of TFL perforators varied from 1.41 to 3.17 per thigh. The mean number of AMT perforators was between 0.59 and 1.3 per thigh. The cumulative assessment of the clinical and anatomical studies showed 456 perforators in 180 TFL flaps (mean, 2.53) and 145 perforators in 162 AMT flaps (mean, 0.90). The mean pedicle length of the TFL and AMT flaps ranged from 7.0 to 9.59 cm and from 7.4 to 11.0 cm, respectively. The mean perforator diameter was similar in both flaps. Conclusion Currently available literature suggests that the TFL flap may be a more reliable alternative when adequate perforators are not found for ALT flap harvest.
[...]
Thieme Medical Publishers 333 Seventh Avenue, New York, NY 10001, USA.
Article in Thieme eJournals:
Table of contents | Abstract | Full text
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2uAGwL6
Development of Targeted Muscle Reinnervation Model in Hind Limb Amputated Rats

J reconstr Microsurg
DOI: 10.1055/s-0038-1639602
Background Targeted muscle reinnervation (TMR) is a novel approach to postamputation neuroma pain; however, this has not been explicitly studied. The purpose of this study was to develop a TMR model in hind limb amputated rats. Methods Ten hind limbs from 5 Sprague Dawley cadaver rats were used. Sciatic nerve, main branches of the sciatic nerve (common peroneal, tibial, sural), motor branches from the sciatic nerve to the biceps femoris and cauda femoris, gluteal nerve and its motor branches to the semimembranosus, and biceps femoris and femoral nerve were dissected to look for consistent nerve anatomy that can be used for TMR in the rat hind limb amputation model. Transfemoral amputation was performed and two types of coaptations were made: common peroneal nerve to motor branch to biceps femoris and tibial nerve to motor branch to semimembranosus. Results The total surgical time for the dissection, amputation, and coaptation of nerves was ∼90 minutes. A total of 100 nerves were dissected in 10 rat hind limbs. Anatomical dissections were straightforward to perform. Anatomy of the dissected nerves was consistent. Hind limb amputations were performed without damaging the target muscles and nerves. Nerve lengths were sufficient for coaptation without any tension. Conclusions To the best of our knowledge, this is the first report on TMR model in hind limb amputated rats. This model will allow for mechanical, electromyography (EMG), and histological analysis for future assessment of neuroma prevention.
[...]
Thieme Medical Publishers 333 Seventh Avenue, New York, NY 10001, USA.
Article in Thieme eJournals:
Table of contents | Abstract | Full text
from #ORL-AlexandrosSfakianakis via ola Kala on Inoreader https://ift.tt/2pYw6A3