Flavonoid
Flavonoids (or bioflavonoids) (from the Latin word flavus meaning yellow, their color in nature) are a class ofplant and fungus secondary metabolites.
Chemically, flavonoids have the general structure of a 15-carbon skeleton, which consists of two phenyl rings (A and B) and heterocyclic ring (C). This carbon structure can be abbreviated C6-C3-C6. According to the IUPACnomenclature,[1][2] they can be classified into:
- flavonoids or bioflavonoids
- isoflavonoids, derived from 3-phenylchromen-4-one (3-phenyl-1,4-benzopyrone) structure
- neoflavonoids, derived from 4-phenylcoumarine (4-phenyl-1,2-benzopyrone) structure
The three flavonoid classes above are all ketone-containing compounds, and as such, are anthoxanthins (flavonesand flavonols). This class was the first to be termed bioflavonoids. The terms flavonoid and bioflavonoid have also been more loosely used to describe non-ketone polyhydroxy polyphenol compounds which are more specifically termed flavanoids. The three cycle or heterocycles in the flavonoid backbone are generally called ring A, B and C. Ring A usually shows a phloroglucinol substitution pattern.
Contents
[hide]Biosynthesis[edit]
Functions of flavonoids in plants[edit]
Flavonoids are widely distributed in plants, fulfilling many functions. Flavonoids are the most important plant pigments for flower coloration, producing yellow or red/blue pigmentation in petals designed to attract pollinator animals. In higher plants, flavonoids are involved in UV filtration, symbiotic nitrogen fixation and floral pigmentation. They may also act as chemical messengers, physiological regulators, and cell cycle inhibitors. Flavonoids secreted by the root of their host plant help Rhizobia in the infection stage of their symbiotic relationship with legumes like peas, beans, clover, and soy. Rhizobia living in soil are able to sense the flavonoids and this triggers the secretion of Nod factors, which in turn are recognized by the host plant and can lead to root hair deformation and several cellular responses such as ion fluxes and the formation of a root nodule. In addition, some flavonoids have inhibitory activity against organisms that cause plant diseases, e.g. Fusarium oxysporum.[3]
Subgroups[edit]
Over 5000 naturally occurring flavonoids have been characterized from various plants. They have been classified according to their chemical structure, and are usually subdivided into the following subgroups (for further reading see[4]):
Anthoxanthins[edit]
Anthoxanthins are divided into two groups:[5]
| Group | Skeleton | Examples | |||
|---|---|---|---|---|---|
| Description | Functional groups | Structural formula | |||
| 3-hydroxyl | 2,3-dihydro | ||||
| Flavone | 2-phenylchromen-4-one | ✗ | ✗ | 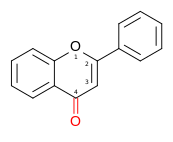 | Luteolin, Apigenin, Tangeritin |
| Flavonol or 3-hydroxyflavone | 3-hydroxy-2-phenylchromen-4-one | ✓ | ✗ |  | Quercetin, Kaempferol, Myricetin, Fisetin, Galangin,Isorhamnetin, Pachypodol, Rhamnazin,Pyranoflavonols, Furanoflavonols, |
Flavanones[edit]
| Group | Skeleton | Examples | |||
|---|---|---|---|---|---|
| Description | Functional groups | Structural formula | |||
| 3-hydroxyl | 2,3-dihydro | ||||
| Flavanone | 2,3-dihydro-2-phenylchromen-4-one | ✗ | ✓ |  | Hesperetin, Naringenin, Eriodictyol,Homoeriodictyol |
Flavanonols[edit]
| Group | Skeleton | Examples | |||
|---|---|---|---|---|---|
| Description | Functional groups | Structural formula | |||
| 3-hydroxyl | 2,3-dihydro | ||||
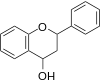
| Flavanonol or 3-Hydroxyflavanone or 2,3-dihydroflavonol | 3-hydroxy-2,3-dihydro-2-phenylchromen-4-one | ✓ | ✓ |  | Taxifolin (orDihydroquercetin),Dihydrokaempferol |
Flavans[edit]
Include flavan-3-ols (flavanols), flavan-4-ols and flavan-3,4-diols.
| Skeleton | Name |
|---|---|
 | Flavan-3-ol (flavanol) |
 | Flavan-4-ol |
 | Flavan-3,4-diol (leucoanthocyanidin) |
- Flavan-3-ols (flavanols)
- Flavan-3-ols use the 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton
- Examples: Catechin (C), Gallocatechin (GC), Catechin 3-gallate (Cg), Gallocatechin 3-gallate (GCg), Epicatechins (Epicatechin (EC)),Epigallocatechin (EGC), Epicatechin 3-gallate (ECg), Epigallocatechin 3-gallate (EGCg)
- Thearubigin
- Proanthocyanidins are dimers, trimers, oligomers, or polymers of the flavanols
Anthocyanidins[edit]
- Anthocyanidins
- Anthocyanidins are the aglycones of anthocyanins; they use the flavylium (2-phenylchromenylium) ion skeleton
- Examples: Cyanidin, Delphinidin, Malvidin, Pelargonidin, Peonidin, Petunidin
Isoflavonoids[edit]
- Isoflavonoids
- Isoflavones use the 3-phenylchromen-4-one skeleton (with no hydroxyl group substitution on carbon at position 2)
Dietary sources[edit]
Flavonoids (specifically flavanoids such as the catechins) are "the most common group of polyphenoliccompounds in the human diet and are found ubiquitously in plants".[6] Flavonols, the original bioflavonoids such as quercetin, are also found ubiquitously, but in lesser quantities. The widespread distribution of flavonoids, their variety and their relatively low toxicity compared to other active plant compounds (for instance alkaloids) mean that many animals, including humans, ingest significant quantities in their diet. Foods with a high flavonoid content include parsley,[7] onions,[7] blueberries and other berries,[7] black tea,[7] green tea and oolong tea,[7] bananas, all citrus fruits, Ginkgo biloba, red wine, sea-buckthorns, anddark chocolate (with a cocoa content of 70% or greater). Further information on dietary sources of flavonoids can be obtained from the US Department of Agriculture flavonoid database.[7]
Parsley[edit]
Parsley, both fresh and dried, contains flavones.[7]
Blueberries[edit]
Blueberries are a dietary source of anthocyanidins.[7][8]
Black tea[edit]
Black tea is a rich source of dietary flavan-3-ols.[7]
Citrus[edit]
The citrus flavonoids include hesperidin (a glycoside of the flavanone hesperetin), quercitrin, rutin (twoglycosides of the flavonol quercetin), and the flavone tangeritin.
Wine[edit]
Cocoa[edit]
Flavonoids exist naturally in cocoa, but because they can be bitter, they are often removed from chocolate, even dark chocolate.[9] Although flavonoids are present in milk chocolate, milk may interfere with their absorption;[10][11] however this conclusion has been questioned.[12]
Peanut[edit]
Peanut (red) skin contains significant polyphenol content, including flavonoids.[13][14]
| Food source | Flavones | Flavonols | Flavanones |
|---|---|---|---|
| Red onion | 0 | 4 - 100 | 0 |
| Parsley, fresh | 24 - 634 | 8 - 10 | 0 |
| Thyme, fresh | 56 | 0 | 0 |
| Lemon juice, fresh | 0 | 0 - 2 | 2 - 175 |
Dietary intake[edit]
Food composition data for flavonoids were provided by the USDA database on flavonoids.[7] In the United States NHANES survey, mean flavonoid intake was 190 mg/d in adults, with flavan-3-ols as the main contributor.[17] In the European Union, based on data from EFSA, mean flavonoid intake was 140 mg/d, although there were considerable differences between individual countries.[16]
The main type of flavonoids consumed in the EU and USA were flavan-3-ols, mainly from tea, while intake of other flavonoids was considerably lower.[16][17]
Research[edit]
Though there is ongoing research into the potential health benefits of individual flavonoids, neither theFood and Drug Administration (FDA) nor the European Food Safety Authority (EFSA) has approved any health claim for flavonoids or approved any flavonoids as pharmaceutical drugs.[18][19][20] Moreover, several companies have been cautioned by the FDA over misleading health claims.[21][22][23][24]
In vitro[edit]
Flavonoids have been shown to have a wide range of biological and pharmacological activities in in vitrostudies. Examples include anti-allergic,[25] anti-inflammatory,[25][26] antioxidant,[26] anti-microbial(antibacterial,[27][28] antifungal,[29][30] and antiviral[29][30]), anti-cancer,[26][31] and anti-diarrheal activities.[32]Flavonoids have also been shown to inhibit topoisomerase enzymes[33][34] and to induce DNA mutations in the mixed-lineage leukemia (MLL) gene in in vitro studies.[35] However, in most of the above cases no follow up in vivo or clinical research has been performed, leaving it impossible to say if these activities have any beneficial or detrimental effect on human health. Biological and pharmacological activities which have been investigated in greater depth are described below.
Antioxidant[edit]
Research at the Linus Pauling Institute and the European Food Safety Authority shows that flavonoids are poorly absorbed in the human body (less than 5%), with most of what is absorbed being quickly metabolized and excreted.[20][36][37] These findings suggest that flavonoids have negligible systemic antioxidant activity, and that the increase in antioxidant capacity of blood seen after consumption of flavonoid-rich foods is not caused directly by flavonoids, but is due to production of uric acid resulting from flavonoid depolymerizationand excretion.[38]
Inflammation[edit]
Inflammation has been implicated as a possible origin of numerous local and systemic diseases, such ascancer,[39] cardiovascular disorders,[40] diabetes mellitus,[41] and celiac disease.[42]
Preliminary studies indicate that flavonoids may affect anti-inflammatory mechanisms via their ability to inhibit reactive oxygen or nitrogen compounds.[43] Flavonoids have also been proposed to inhibit the pro-inflammatory activity of enzymes involved in free radical production, such as cyclooxygenase, lipoxygenaseor inducible nitric oxide synthase,[43][44] and to modify intracellular signaling pathways in immune cells,[43] or in brain cells after a stroke.[45]
Procyanidins, a class of flavonoids, have been shown in preliminary research to have anti-inflammatory mechanisms including modulation of thearachidonic acid pathway, inhibition of gene transcription, expression and activity of inflammatory enzymes, as well as secretion of anti-inflammatory mediators.[46]
Cancer[edit]
Clinical studies investigating the relationship between flavonoid consumption and cancer prevention/development are conflicting for most types of cancer, probably because most studies are retrospective in design and use a small sample size.[47] Two apparent exceptions are gastric carcinomaand smoking-related cancers. Dietary flavonoid intake is associated with reduced gastric carcinoma risk in women,[48] and reduced aerodigestive tract cancer risk in smokers.[49]
Cardiovascular diseases[edit]
Among the most intensively studied of general human disorders possibly affected by dietary flavonoids, preliminary cardiovascular diseaseresearch has revealed the following mechanisms under investigation in patients or normal subjects:[50][51][52][53][54]
- inhibit coagulation, thrombus formation or platelet aggregation
- reduce risk of atherosclerosis
- reduce arterial blood pressure and risk of hypertension
- reduce oxidative stress and related signaling pathways in blood vessel cells
- modify vascular inflammatory mechanisms
- improve endothelial and capillary function
- modify blood lipid levels
- regulate carbohydrate and glucose metabolism
- modify mechanisms of aging
Listed on the clinical trial registry of the US National Institutes of Health (July 2016) are 48 human studies completed or underway to study the dietary effects of plant flavonoids on cardiovascular diseases.[55]
However, population-based studies have failed to show a strong beneficial effect[56] which might be due to the considerably lower intake in the habitual diet of those investigated.
Antibacterial[edit]
Flavonoids have been shown to have (a) direct antibacterial activity, (b) synergistic activity with antibiotics, and (c) the ability to suppress bacterialvirulence factors in numerous in vitro and a limited number of in vivo studies.[27][57] Noteworthy among the in vivo studies[58][59][60] is the finding that oral quercetin protects guinea pigs against the Group 1 carcinogen Helicobacter pylori.[60] Researchers from the European Prospective Investigation into Cancer and Nutrition have speculated this may be one reason why dietary flavonoid intake is associated with reduced gastric carcinoma risk in European women.[61] Additional in vivo and clinical research is needed to determine if flavonoids could be used as pharmaceutical drugs for the treatment of bacterial infection, or whether dietary flavonoid intake offers any protection against infection.
Synthesis, detection, quantification, and semi-synthetic alterations[edit]
Color spectrum[edit]
Flavonoid synthesis in plants is induced by light color spectrums at both high and low energy radiations. Low energy radiations are accepted byphytochrome, while high energy radiations are accepted by carotenoids, flavins, cryptochromes in addition to phytochromes. Thephotomorphogenic process of phytochome-mediated flavonoid biosynthesis has been observed in Amaranthus, barley, maize, Sorghum and turnip. Red light promotes flavonoid synthesis.[62]
Availability through microorganisms[edit]
Several recent research articles have demonstrated the efficient production of flavonoid molecules from genetically engineered microorganisms.[63][64][65]
Tests for detection[edit]
- Shinoda test
Four pieces of magnesium filings are added to the ethanolic extract followed by few drops of concentrated hydrochloric acid. A pink or red colour indicates the presence of flavonoid.[66] Colours varying from orange to red indicated flavones, red to crimson indicated flavonoids, crimson to magenta indicated flavonones.
- Sodium hydroxide test
About 5 mg of the compound is dissolved in water, warmed and filtered. 10% aqueous sodium hydroxide is added to 2 ml of this solution. This produces a yellow coloration. A change in color from yellow to colorless on addition of dilute hydrochloric acid is an indication for the presence of flavonoids.[67]
- p-Dimethylaminocinnamaldehyde test
A colorimetric assay based upon the reaction of A-rings with the chromogen p-dimethylaminocinnamaldehyde (DMACA) has been developed for flavanoids in beer that can be compared with the vanillin procedure.[68]
Quantification[edit]
Lamaison and Carnet have designed a test for the determination of the total flavonoid content of a sample (AlCI3 method). After proper mixing of the sample and the reagent, the mixture is incubated for 10 minutes at ambient temperature and the absorbance of the solution is read at 440 nm. Flavonoid content is expressed in mg/g of quercetin.[69]
Semi-synthetic alterations[edit]
Immobilized Candida antarctica lipase can be used to catalyze the regioselective acylation of flavonoids.[70]
See also[edit]
- Phytochemical
- List of antioxidants in food
- List of phytochemicals in food
- Phytochemistry
- Secondary metabolites
- Homoisoflavonoids, related chemicals with a 16 carbons skeleton
References[edit]
- ^ McNaught, Alan D; Wilkinson, Andrew; IUPAC (1997), "IUPAC Compendium of Chemical Terminology", IUPAC Compendium of Chemical Terminology (2 ed.), Oxford: Blackwell Scientific, ISBN 0-9678550-9-8, doi:10.1351/goldbook.F02424
- ^ "The Gold Book". 2009. ISBN 0-9678550-9-8.doi:10.1351/goldbook. Retrieved 16 September 2012.
|chapter=ignored (help) - ^ Galeotti, F; Barile, E; Curir, P; Dolci, M; Lanzotti, V (2008). "Flavonoids from carnation (Dianthus caryophyllus) and their antifungal activity". Phytochemistry Letters. 1: 44–48.doi:10.1016/j.phytol.2007.10.001.
- ^ Ververidis F, Trantas E, Douglas C, Vollmer G, Kretzschmar G, Panopoulos N (October 2007). "Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health". Biotechnology Journal. 2(10): 1214–34. PMID 17935117. doi:10.1002/biot.200700084.
- ^ Isolation of a UDP-glucose: Flavonoid 5-O-glucosyltransferase gene and expression analysis of anthocyanin biosynthetic genes in herbaceous peony (Paeonia lactiflora Pall.). Da Qiu Zhao, Chen Xia Han, Jin Tao Ge and Jun Tao, Electronic Journal of Biotechnology, 15 November 2012, Volume 15, Number 6, doi:10.2225/vol15-issue6-fulltext-7
- ^ Spencer JP (2008). "Flavonoids: modulators of brain function?". British Journal of Nutrition. 99: ES60–77. PMID 18503736.doi:10.1017/S0007114508965776.
- ^ a b c d e f g h i j USDA's Database on the Flavonoid Content
- ^ Ayoub M, de Camargo AC, Shahidi F (2016). "Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals". Food Chemistry. 197 (Part A): 221–232.doi:10.1016/j.foodchem.2015.10.107.
- ^ "The devil in the dark chocolate". Lancet. 370 (9605): 2070. 2007.PMID 18156011. doi:10.1016/S0140-6736(07)61873-X.
- ^ Serafini M, Bugianesi R, Maiani G, Valtuena S, De Santis S, Crozier A (2003). "Plasma antioxidants from chocolate". Nature. 424 (6952): 1013. Bibcode:2003Natur.424.1013S. PMID 12944955.doi:10.1038/4241013a.
- ^ Serafini M, Bugianesi R, Maiani G, Valtuena S, De Santis S, Crozier A (2003). "Nutrition: milk and absorption of dietary flavanols". Nature.424 (6952): 1013. Bibcode:2003Natur.424.1013S. PMID 12944955.doi:10.1038/4241013a.
- ^ Roura E, et al. (2007). "Milk Does Not Affect the Bioavailability of Cocoa Powder Flavonoid in Healthy Human" (PDF). Ann Nutr Metab.51: 493–498. doi:10.1159/000111473.
- ^ de Camargo AC, Regitano-d'Arce MA, Gallo CR, Shahidi F (2015)."Gamma-irradiation induced changes in microbiological status, phenolic profile and antioxidant activity of peanut skin". Journal of Functional Foods. 12: 129–143. doi:10.1016/j.jff.2014.10.034.
- ^ Chukwumah Y, Walker LT, Verghese M (2009). "Peanut skin color: a biomarker for total polyphenolic content and antioxidative capacities of peanut cultivars". Int J Mol Sci. 10 (11): 4941–52. PMC 2808014
 .PMID 20087468. doi:10.3390/ijms10114941.
.PMID 20087468. doi:10.3390/ijms10114941. - ^ "Flavonoids - Linus Pauling Institute - Oregon State University". Retrieved 26 February 2016.
- ^ a b c d Vogiatzoglou, A; Mulligan, A. A.; Lentjes, M. A.; Luben, R. N.; Spencer, J. P.; Schroeter, H; Khaw, K. T.; Kuhnle, G. G. (2015)."Flavonoid intake in European adults (18 to 64 years)". PLoS ONE. 10(5): e0128132. PMC 4444122
 . PMID 26010916.doi:10.1371/journal.pone.0128132.
. PMID 26010916.doi:10.1371/journal.pone.0128132. - ^ a b Chun, O. K.; Chung, S. J.; Song, W. O. (2007). "Estimated dietary flavonoid intake and major food sources of U.S. Adults". The Journal of Nutrition. 137 (5): 1244–52. PMID 17449588.
- ^ "FDA approved drug products". US Food and Drug Administration. Retrieved 8 November 2013.
- ^ "Health Claims Meeting Significant Scientific Agreement". US Food and Drug Administration. Retrieved 8 November 2013.
- ^ a b EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA)2, 3 European Food Safety Authority (EFSA), Parma, Italy (2010)."Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to Article 13(1) of Regulation (EC) No 1924/20061"(PDF). EFSA Journal. 8 (2): 1489. doi:10.2903/j.efsa.2010.1489.
- ^ "Inspections, Compliance, Enforcement, and Criminal Investigations (Flavonoid Sciences)". US Food and Drug Administration. Retrieved8 November 2013.
- ^ "Inspections, Compliance, Enforcement, and Criminal Investigations (Unilever, Inc.)". US Food and Drug Administration. Retrieved25 October 2013.
- ^ "Lipton green tea is a drug". NutraIngredients-USA.com. Retrieved25 October 2013.
- ^ "Fruits Are Good for Your Health? Not So Fast: FDA Stops Companies From Making Health Claims About Foods". TheDailyGreen.com. Retrieved 25 October 2013.
- ^ a b Yamamoto Y, Gaynor RB (2001). "Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer". Journal of Clinical Investigation. 107 (2): 135–42.PMC 199180
 . PMID 11160126. doi:10.1172/JCI11914.
. PMID 11160126. doi:10.1172/JCI11914. - ^ a b c Cazarolli LH, Zanatta L, Alberton EH, Figueiredo MS, Folador P, Damazio RG, Pizzolatti MG, Silva FR (2008). "Flavonoids: Prospective Drug Candidates". Mini-Reviews in Medicinal Chemistry. 8 (13): 1429–1440. PMID 18991758. doi:10.2174/138955708786369564.
- ^ a b Cushnie TP, Lamb AJ (2011). "Recent advances in understanding the antibacterial properties of flavonoids". International Journal of Antimicrobial Agents. 38 (2): 99–107. PMID 21514796.doi:10.1016/j.ijantimicag.2011.02.014.
- ^ Manner S, Skogman M, Goeres D, Vuorela P, Fallarero A (2013)."Systematic exploration of natural and synthetic flavonoids for the inhibition of Staphylococcus aureus biofilms". International Journal of Molecular Sciences. 14 (10): 19434–19451. PMC 3821565
 .PMID 24071942. doi:10.3390/ijms141019434.
.PMID 24071942. doi:10.3390/ijms141019434. - ^ a b Cushnie TP, Lamb AJ (2005). "Antimicrobial activity of flavonoids" (PDF). International Journal of Antimicrobial Agents. 26(5): 343–356. PMID 16323269.doi:10.1016/j.ijantimicag.2005.09.002.
- ^ a b Friedman M (2007). "Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas". Molecular Nutrition and Food Research. 51 (1): 116–134. PMID 17195249.doi:10.1002/mnfr.200600173.
- ^ de Sousa RR, Queiroz KC, Souza AC, Gurgueira SA, Augusto AC, Miranda MA, Peppelenbosch MP, Ferreira CV, Aoyama H (2007). "Phosphoprotein levels, MAPK activities and NFkappaB expression are affected by fisetin". J Enzyme Inhib Med Chem. 22 (4): 439–444.PMID 17847710. doi:10.1080/14756360601162063.
- ^ Schuier M, Sies H, Illek B, Fischer H (2005). "Cocoa-related flavonoids inhibit CFTR-mediated chloride transport across T84 human colon epithelia". J. Nutr. 135 (10): 2320–5. PMID 16177189.
- ^ Esselen M, Fritz J, Hutter M, Marko D (2009). "Delphinidin Modulates the DNA-Damaging Properties of Topoisomerase II Poisons". Chemical Research in Toxicology. 22 (3): 554–64. PMID 19182879.doi:10.1021/tx800293v.
- ^ Bandele OJ, Clawson SJ, Osheroff N (2008). "Dietary polyphenols as topoisomerase II poisons: B-ring substituents determine the mechanism of enzyme-mediated DNA cleavage enhancement". Chemical Research in Toxicology. 21 (6): 1253–1260. PMC 2737509
 .PMID 18461976. doi:10.1021/tx8000785.
.PMID 18461976. doi:10.1021/tx8000785. - ^ Barjesteh van Waalwijk van Doorn-Khosrovani S, Janssen J, Maas LM, Godschalk RW, Nijhuis JG, van Schooten FJ (2007). "Dietary flavonoids induce MLL translocations in primary human CD34+ cells".Carcinogenesis. 28 (8): 1703–9. PMID 17468513.doi:10.1093/carcin/bgm102.
- ^ Lotito SB, Frei B (2006). "Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon?". Free Radic. Biol. Med. 41 (12): 1727–46. PMID 17157175.doi:10.1016/j.freeradbiomed.2006.04.033.
- ^ Williams RJ, Spencer JP, Rice-Evans C (2004). "Flavonoids: antioxidants or signalling molecules?". Free Radical Biology & Medicine.36 (7): 838–49. PMID 15019969.doi:10.1016/j.freeradbiomed.2004.01.001.
- ^ Stauth D (5 March 2007). "Studies force new view on biology of flavonoids". EurekAlert!, Adapted from a news release issued by Oregon State University.
- ^ Ravishankar D, Rajora AK, Greco F, Osborn HM (2013). "Flavonoids as prospective compounds for anti-cancer therapy". The International Journal of Biochemistry & Cell Biology. 45 (12): 2821–2831.PMID 24128857. doi:10.1016/j.biocel.2013.10.004.
- ^ Manach C, Mazur A, Scalbert A (2005). "Polyphenols and prevention of cardiovascular diseases". Current opinion in lipidology. 16 (1): 77–84.PMID 15650567. doi:10.1097/00041433-200502000-00013.
- ^ Babu PV, Liu D, Gilbert ER (2013). "Recent advances in understanding the anti-diabetic actions of dietary flavonoids". The Journal of Nutritional Biochemistry. 24 (11): 1777–1789. PMC 3821977
 . PMID 24029069. doi:10.1016/j.jnutbio.2013.06.003.
. PMID 24029069. doi:10.1016/j.jnutbio.2013.06.003. - ^ Ferretti G, Bacchetti T, Masciangelo S, Saturni L (2012). "Celiac Disease, Inflammation and Oxidative Damage: A Nutrigenetic Approach". Nutrients. 4 (12): 243–257. PMC 3347005
 .PMID 22606367. doi:10.3390/nu4040243.
.PMID 22606367. doi:10.3390/nu4040243. - ^ a b c Izzi V, Masuelli L, Tresoldi I, Sacchetti P, Modesti A, Galvano F, Bei R (2012). "The effects of dietary flavonoids on the regulation of redox inflammatory networks". Frontiers in bioscience (Landmark edition). 17(7): 2396–2418. PMID 22652788. doi:10.2741/4061.
- ^ Gomes A, Couto D, Alves A, Dias I, Freitas M, Porto G, Duarte JA, Fernandes E (2012). "Trihydroxyflavones with antioxidant and anti-inflammatory efficacy". BioFactors. 38 (5): 378–386. PMID 22806885.doi:10.1002/biof.1033.
- ^ Chang CF, Cho S, Wang J (Apr 2014). "(-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways". Ann Clin Transl Neurol. 1 (4): 258–271. PMC 3984761
 . PMID 24741667.doi:10.1002/acn3.54.
. PMID 24741667.doi:10.1002/acn3.54. - ^ Martinez-Micaelo N, González-Abuín N, Ardèvol A, Pinent M, Blay MT (2012). "Procyanidins and inflammation: Molecular targets and health implications". BioFactors. 38 (4): 257–265. PMID 22505223.doi:10.1002/biof.1019.
- ^ Romagnolo DF, Selmin OI (2012). "Flavonoids and cancer prevention: a review of the evidence". J Nutr Gerontol Geriatr. 31 (3): 206–38.PMID 22888839. doi:10.1080/21551197.2012.702534.
- ^ González CA, Sala N, Rokkas T (2013). "Gastric cancer: epidemiologic aspects". Helicobacter. 18 (Supplement 1): 34–38.PMID 24011243. doi:10.1111/hel.12082.
- ^ Woo HD, Kim J (2013). "Dietary flavonoid intake and smoking-related cancer risk: a meta-analysis". PLoS ONE. 8 (9): e75604.Bibcode:2013PLoSO...875604W. PMC 3777962
 .PMID 24069431. doi:10.1371/journal.pone.0075604.
.PMID 24069431. doi:10.1371/journal.pone.0075604. - ^ Higdon, J; Drake, V; Frei, B (March 2009). "Non-Antioxidant Roles for Dietary Flavonoids: Reviewing the relevance to cancer and cardiovascular diseases". Nutraceuticals World. Rodman Media. Retrieved 24 November 2013.
- ^ van Dam RM, Naidoo N, Landberg R (2013). "Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases".Current Opinion in Lipidology. 24 (1): 25–33. PMID 23254472.doi:10.1097/MOL.0b013e32835bcdff.
- ^ Tangney CC, Rasmussen HE (2013). "Polyphenols, Inflammation, and Cardiovascular Disease". Current Atherosclerosis Reports. 15 (5): 324. PMC 3651847
 . PMID 23512608. doi:10.1007/s11883-013-0324-x.
. PMID 23512608. doi:10.1007/s11883-013-0324-x. - ^ Siasos G, Tousoulis D, Tsigkou V, Kokkou E, Oikonomou E, Vavuranakis M, Basdra EK, Papavassiliou AG, Stefanadis C (2013). "Flavonoids in atherosclerosis: An overview of their mechanisms of action". Current medicinal chemistry. 20 (21): 2641–2660.PMID 23627935. doi:10.2174/0929867311320210003.
- ^ Cappello, AR, Dolce V, Iacopetta D, Martello M, Fiorillo M, Curcio R, Muto L, Dhanyalayam D. (2015). "Bergamot (Citrus bergamia Risso) Flavonoids and Their Potential Benefits in Human Hyperlipidemia and Atherosclerosis: an Overview". Mini-Reviews in Medicinal Chemistry. 16(8): 1–11. PMID 26156545.doi:10.2174/1389557515666150709110222.
- ^ Search Results. "Flavonoids in cardiovascular disease clinical trials". Clinicaltrials.gov. US National Institutes of Health. RetrievedNovember 24, 2013.
- ^ Wang X; Ouyang YY; Liu J; Zhao G (January 2014). "Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies". The British journal of nutrition. 111 (1): 1–11.PMID 23953879. doi:10.1017/S000711451300278X.
- ^ Taylor PW, Hamilton-Miller JM, Stapleton PD (2005). "Antimicrobial properties of green tea catechins". Food Science and Technology Bulletin. 2 (7): 71–81. PMC 2763290
 . PMID 19844590.doi:10.1616/1476-2137.14184.
. PMID 19844590.doi:10.1616/1476-2137.14184. - ^ Choi O, Yahiro K, Morinaga N, Miyazaki M, Noda M (2007). "Inhibitory effects of various plant polyphenols on the toxicity of Staphylococcal alpha-toxin". Microbial Pathogenesis. 42 (5–6): 215–224.PMID 17391908. doi:10.1016/j.micpath.2007.01.007.
- ^ Oh DR, Kim JR, Kim YR (2010). "Genistein inhibits Vibrio vulnificus adhesion and cytotoxicity to HeLa cells". Archives of Pharmacal Research. 33 (5): 787–792. PMID 20512479. doi:10.1007/s12272-010-0520-y.
- ^ a b González-Segovia R, Quintanar JL, Salinas E, Ceballos-Salazar R, Aviles-Jiménez F, Torres-López J (2008). "Effect of the flavonoid quercetin on inflammation and lipid peroxidation induced by Helicobacter pylori in gastric mucosa of guinea pig". Journal of Gastroenterology. 43(6): 441–447. PMID 18600388. doi:10.1007/s00535-008-2184-7.
- ^ Zamora-Ros R, Agudo A, Luján-Barroso L, Romieu I, Ferrari P, Knaze V, Bueno-de-Mesquita HB, Leenders M, Travis RC, Navarro C, Sánchez-Cantalejo E, Slimani N, Scalbert A, Fedirko V, Hjartåker A, Engeset D, Skeie G, Boeing H, Förster J, Li K, Teucher B, Agnoli C, Tumino R, Mattiello A, Saieva C, Johansson I, Stenling R, Redondo ML, Wallström P, Ericson U, Khaw KT, Mulligan AA, Trichopoulou A, Dilis V, Katsoulis M, Peeters PH, Igali L, Tjønneland A, Halkjær J, Touillaud M, Perquier F, Fagherazzi G, Amiano P, Ardanaz E, Bredsdorff L, Overvad K, Ricceri F, Riboli E, González CA (2012). "Dietary flavonoid and lignan intake and gastric adenocarcinoma risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study". American Journal of Clinical Nutrition. 96 (6): 1398–1408. PMID 23076618.doi:10.3945/ajcn.112.037358.
- ^ Sinha, Rajiv Kumar (2004-01-01). Modern Plant Physiology. CRC Press. p. 457. ISBN 9780849317149.
- ^ Hwang EI, Kaneko M, Ohnishi Y, Horinouchi S (May 2003)."Production of plant-specific flavanones by Escherichia coli containing an artificial gene cluster". Appl. Environ. Microbiol. 69 (5): 2699–706.PMC 154558
 . PMID 12732539. doi:10.1128/AEM.69.5.2699-2706.2003.
. PMID 12732539. doi:10.1128/AEM.69.5.2699-2706.2003. - ^ Trantas E, Panopoulos N, Ververidis F (2009). "Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae". Metabolic Engineering. 11 (6): 355–366. PMID 19631278.doi:10.1016/j.ymben.2009.07.004.
- ^ Ververidis F, Trantas E, Douglas C, Vollmer G, Kretzschmar G, Panopoulos N (2007). "Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part II: Reconstruction of multienzyme pathways in plants and microbes". Biotechnology Journal.2 (10): 1235–49. PMID 17935118. doi:10.1002/biot.200700184.
- ^ Yisa, Jonathan (2009). "Phytochemical Analysis and Antimicrobial Activity Of Scoparia Dulcis and Nymphaea Lotus". Australian Journal of Basic and Applied Sciences. 3 (4): 3975–3979.
- ^ Bello IA, Ndukwe GI, Audu OT, Habila JD (2011). "A bioactive flavonoid from Pavetta crassipes K. Schum". Organic and Medicinal Chemistry Letters. 1 (1): 14. PMC 3305906
 . PMID 22373191.doi:10.1186/2191-2858-1-14.
. PMID 22373191.doi:10.1186/2191-2858-1-14. - ^ A new colourimetric assay for flavonoids in pilsner beers. Jan A. Delcour and Didier Janssens de Varebeke, Journal of the Institute of Brewing, January–February 1985, Volume 91, Issue 1, pages 37–40,doi:10.1002/j.2050-0416.1985.tb04303.x
- ^ Lamaison, JL; Carnet, A (1991). "Teneurs en principaux flavonoides des fleurs de Cratageus monogyna Jacq et de Cratageus Laevigata (Poiret D.C) en Fonction de la vegetation". Plantes Medicinales Phytotherapie. 25: 12–16.
- ^ Passicos E, Santarelli X, Coulon D (2004). "Regioselective acylation of flavonoids catalyzed by immobilized Candida antarctica lipase under reduced pressure". Biotechnol Lett. 26 (13): 1073–1076.PMID 15218382. doi:10.1023/B:BILE.0000032967.23282.15.
Further reading[edit]
- Andersen, Ø.M. / Markham, K.R. (2006). Flavonoids: Chemistry, Biochemistry and Applications. CRC Press. ISBN 978-0-8493-2021-7
- Grotewold, Erich (2007). The Science of Flavonoids. Springer. ISBN 978-0-387-74550-3
- Comparative Biochemistry of the Flavonoids, by J.B. Harborne, 1967 (Google Books)
- The systematic identification of flavonoids, by T.J. Mabry, K.R. Markham and M.B. Thomas, 1970, doi:10.1016/0022-2860(71)87109-0
External links[edit]
Databases[edit]
Anapafseos 5 . Agios Nikolaos
Crete.Greece.72100
2841026182








Complanatuside A, a flavonoid compound, is isolated from Astragalus complanatus and Lysimachia christinae. Astragalus complanatus R.Brown is a widely used herbal material in traditional Chinese medicine. The total flavonoid (TF) is an active fraction extracted from seeds of Astragalus complanatus R.Brown. Previous studies performed in animals have demonstrated the antihypertensive efficacy of the TF. Complanatuside A
ΑπάντησηΔιαγραφή